AbstractEndoscopic submucosal dissection (ESD) has enabled en bloc resection of early stage gastrointestinal tumors with negligible risk of lymph node metastasis, regardless of tumor size, location, and shape. However, ESD is a relatively difficult technique compared with conventional endoscopic mucosal resection, requiring a longer procedure time and potentially causing more complications. For safe and reproducible procedure of ESD, the appropriate dissection of the ramified vascular network in the level of middle submucosal layer is required to reach the avascular stratum just above the muscle layer. The horizontal approach to maintain the appropriate depth for dissection beneath the vascular network enables treatment of difficult cases with large vessels and severe fibrosis. The most important aspect of ESD is the precise evaluation of curability. This approach can also secure the quality of the resected specimen with enough depth of the submucosal layer.
INTRODUCTIONEndoscopic submucosal dissection (ESD) was developed based on endoscopic resection with local injection of hypertonic saline-epinephrine for a more reliable endoscopic resection.1-9 Endoscopic mucosal resection (EMR) was the application of polypectomy using a snare, whereas, ESD was the application of surgical techniques using various endo-knives to perform a complete circumferential mucosal incision and submucosal dissection under direct vision. Theoretically, ESD is an innovative method which can enable endoscopic en bloc resection regardless of size, location, and shape of lesion. En bloc R0 resection rate of EMR was reported in up to 40% to 50% of cases for 2 to 3 cm lesions without ulcer scar,10-13 whereas for ESD in high volume centers en bloc rates were over 95%, even for lesions larger than 10 cm and those with ulcer scars.14-20 However, with ESD there is concern about technical difficulties, higher incidence of complications, and longer procedure time, besides which EMR is a relatively easy, safe, and swift procedure.17,19,21-25 The evaluation of curability is currently important because the criteria for endoscopic resection were expanded to large mucosal cancer with ulcer scar and slightly invasive submucosal cancer. ESD is no more than local resection, though it enables resection regardless of size, therefore the indication for ESD must be lesions with a negligible risk of lymph node metastasis. At present, there is no certain modality to preoperatively diagnose the negativity of lymph node metastasis. The curability is presently decided based on the histopathological diagnosis of the resected specimen (Table 1).26,27 Therefore, a high quality resected specimen with enough depth of submucosal layer is required for the precise evaluation of invasion depth and form, and also for lympho-vascular invasion. We have reported that the appropriate dissection of the ramified vascular network in the level of middle submucosal layer to reach the avascular stratum just above the muscle layer enables safe and reproducible procedure of ESD and the horizontal approach to maintain the appropriate depth for dissection beneath the vascular network enables treatment of difficult cases with large vessels and severe fibrosis, and also demonstrated these approaches could lead to obtaining a high quality resected specimen.28-32 In this article, we show the principle of quality controlled ESD.
The factors of difficulty in ESD and the appropriate depth for submucosal dissectionThe procedure of ESD consists of mucosal incision and submucosal dissection. Both approaches must be performed at a shallower level than muscle layer. However, there is no clear standard to the depth of mucosal incision and submucosal dissection, and there exists only the undefined suggestion that the incision should not be too shallow and the dissection should be to the lower third of submucosal layer. These are relative standards and the actual approach can be shallow or deep according to the amount of local injection solution, even if the incision and dissection were carried out by same distance from the muscular layer. There was a suggestion that the dissection in the shallow level of submucosal layer would prevent the perforation in the initial period of ESD; however this approach has not only a high risk of the positive vertical margin of tumor but also leads to loss of the important findings mentioned above. We focused on the existence of horizontal ramified vascular network in the submucosal layer, and defined clearly the appropriate incision depth to be beneath muscularis mucosa and above vascular network, and the appropriate dissection depth to be beneath vascular network and above muscle layer.
The difficult case for ESDESD would be relatively easy if there was no bleeding. Intraoperative bleeding is not judged as a complication when the patient's hemoglobin level does not decrease by 2 g/dL and does not require blood transfusion. However, cases with frequent bleeding and the need for frequent hemostasis are typically difficult cases for ESD. The cases that easily bleed in the incision will also easily bleed in the dissection.
The worst postulated sequence of possible events of ESDIn a "worst case," the bleeding occurs just after the beginning of incision. The hemostasis is quite difficult and the incision is finally finished after frequent hemostasis. However, the bleeding occurs again immediately after the beginning of dissection. The edge of incision is gradually coagulated and burned to be tough, and the entry of the devices into the submucosal layer is impossible beneath the incised mucosa, like a shell closing its husk. The entry into the submucosal layer is carried out from other mucosa, however massive bleeding occurs during the dissection. The lesion is damaged and well-controlled ESD is discontinued. Piecemeal EMR and careless ESD which does not recognize where to dissect lead to perforation in the muscle layer. These are common worst possible events of ESD (Fig. 1).
The structure of vessels in the gastrointestinal luminal wall and the appropriate depth of dissectionThe difficulty of ESD is considered to arise from ignorance of the structure of vessels in the gastrointestinal luminal wall and appropriate depth of dissection. These vessels normally penetrate the muscle layer vertically and then inflow horizontally at the level of middle submucosal layer forming the ramified vascular network (Fig. 2A). In areas with a high density of vessels, the faciae-like layer is formed with the ramified vascular network and the perivascular fibrotic tissue, and the layer containing fewer vessels and fibrotic tissue exists below the faciae and just above the muscular propria (Fig. 2B). Smooth dissection with less hemorrhage is enabled by maintaining the appropriate depth of dissection at the layer with fewer vessels and fibrotic tissue and by coagulating the penetrating vessels before dissection (Fig. 3C). The ramified vessel network in the submucosal layer also connects the edges of incision. Certain dissection of this network allows entry into the appropriate depth of submucosal dissection by broadening the groove made by mucosal incision (Fig. 3A, B). In the procedure of ESD, the most important step is the approach to this appropriate avascular submucosal layer between muscle layer and vessel network followed by the dissection of the vascular network at the edges of mucosal incision.
On the contrary, the difficulty of ESD is caused by straying into this ramified vessel network. The bleeding points are hard to recognize in the hemorrhage at the mucosal incision. The edge of the incised mucosa is not expanded yet, and the bleeding is hard to be grasped by hemostatic forceps. Multiple ineffective attempts at hemostasis will burn the submucosal tissue to shrink and toughen, and these processes make the dissection more difficult. The edges of dissection are tightly narrowed, and the irresponsible dissection causes reiteration of the bleeding. Accidental entry into the appropriate layer for dissection will even frequently hurt the vessel network and penetrating vessels and cause repetitive bleeding. The repeated bleeding and hemostasis will disturb the transparency of the submucosal layer and raise the risk of the damage of the lesion. These scripts are the mechanism of the worst postulated sequence of possible events of ESD that was mentioned above.
The difference of the density of the vessels and the level of difficultyThe density of the vessels differs according to location, and causes differences in the level of difficulty. As shown in the Fig. 4A, the extremely characteristic muscle layers named the oblique muscle layers are symmetrically seen in the anterior/posterior regions of the gastric body. The density and thickness of the vessels in the gastric antrum overwhelmingly differ from those in the gastric body. In the antrum, the density of vessel in the submucosal layer is low, and the fibrosis is also minimal, and these allow easy mucosal incision and submucosal dissection (Fig. 4Ba). In the lesser curvature of gastric body where the oblique muscle layers do not exist, as the blood vessels do not diverge frequently and the density of blood vessels is low as in the gastric antrum, the procedure is rather easy if the large penetrating vessels are not hurt by mistake (Fig. 4Bb). On the other hand, in the anterior/posterior walls of gastric body, where the oblique muscle layers exist, the greater curvature of gastric body and lower rectum, the density of blood vessels is high, the diverged vessel network is inevitably hurt, if careful attention is not paid to the depth of mucosal incision and submucosal dissection (Figs. 4Bc, 5). In the esophagus, the density of vessels is relatively high, and the vessels in the submucosal layer tend to extend longitudinally. The horizontal mucosal incision and dissection frequently cause intraoperative bleeding. The blood vessels are relatively thin; however, the edge of incised mucosa will not be expanded without dissection of this vessel network. The volume of blood loss is not great in quantity, but the field of vision becomes extremely poor because the lumen of esophagus is narrow and the procedure is easily affected by the submergence of lesion. The muscle layer of esophagus is extremely thin, and therefore a sensitive procedure is required. Maintenance of a clear field of vision is important by consistently treating the vessels not to bleed.
THE TECHNIQUE TO REACH THE APPROPRIATE DEPTH FOR SUBMUCOSAL DISSECTION AND THE CHARACTERISTICS OF THE RESECTED SPECIMEN (OBTAINED BY THE TECHNIQUE)The technical tip to reach the appropriate depth of submucosal dissectionAn important subject is how to treat the vessel network
properly in the submucosal layer. The approach to dissect the vessel network after the shallow mucosal incision is critical not to damage the blood vessels.
The ramified vessel network can be seen through when the mucosal incision was kept shallow just below the muscularis mucosae. Intraoperative bleeding seldom occurs when the submusocal dissection is carried out between the blood vessels, while the large vessels are pre-coagulated by hemostatic forceps with soft coagulation mode and both edges of small vessels are pre-coagulated by the endo-knife itself with soft, forced, or spray coagulation mode then dissected with forced or swift coagulation mode. The "arm-cut" technique is recommended for the dissection of vessels, which is to scoop up from the bottom of the vessel into the lumen. This manipulation is in the direction to go away from the muscle layer and the procedure enables taking enough time for coagulation, therefore intraoperative bleeding rarely occurs. The most appropriate tension can be also applied to the object because the tough vessels and peripheral fibrosis are dissected vertically.
Once the vessel network is dissected, the edges of mucosal incision are quite easily expanded. The transparent hood itself can shortly get into the submucosal layer after timely additional mucosal incision and dissection of the vessel network has enlarged the expanded area. The blood-less transparent submucosal layer can be observed. The most important step is to form a mucosal flap after obtaining this appropriate depth of submucosal dissection in the procedure of ESD. We have tried various approaches such as using the IT knife combined with Needle knife30 and also using the Flex knife combined with local injection of hyaluronic acid,33 then finally have developed the technique using Flush knife. Flush knife is an extremely useful endo-knife to form a mucosal flap by reaching the appropriate depth of submucosal dissection. This knife enables shallow mucosal dissection just below the muscularis mucosae, therefore the bleeding during mucosal incision rarely occurs. The water-jet emitting function of Flush knife also enables safe exposure of the vessel network by additional local injection between the vessels to identify and raise the vessel network. The tip of sheath of this knife is used as the protector and clasp, and the "arm cut" from the bottom of vessel network is easily performed still protecting the muscle layer.30,32,34 The newly developed ball tipped Flush knife (Flush knife BT) can scoop up the vessel network and also has significantly improved ability of pre-coagulation and hemostasis than the conventional Flush knife;35 therefore, Flush knife BT enables effective dissection of vessel network (Fig. 6).32
The technical tip to maintain the appropriate depth of submucosal dissectionOnce the blood vessels are damaged by mistake, the field of vision is lost and subsequent procedure becomes difficult, even after the appropriate depth of dissection has been obtained. The only measure is to dissect with preoperative recognition of the vessels and muscular layer while maintaining the appropriate depth of submucosal dissection followed by additional local injection beneath the vessel network. Especially, it is always necessary to distinguish the recognized vessels whether these are horizontal small branches of the vessel network or vertical large branches of the penetrating vessels. If the vessels are horizontal branches of the vessel network, the dissection should be done below these, while if these are vertical branches of penetrating vessels, the dissection at the base of the vessels should be done after the exposure and pre-coagulation. The muscle layer is absent at the inlet of the large vessel (Fig. 5B), therefore attention is required for perforation in cases with difficult hemostasis for bleeding by mistake. Especially in the rectum, the sparse fibers of muscle layer are piled up in the shape of roofing tiles, and both sides of muscle layer at the inlet are extended out in the shape of wedge. In the esophagus, the muscle layer is sometimes loose, so disorientation into the fibers of muscle layer must be prevented by always taking the dissection between the muscle layer and the vessel network into consideration.
The structure of blood vessels in the mucosa of the gastrointestinal tract is similar to the trunk and branch of the Wisteria trellis. Alternatively, the structure of blood vessels in the submucosal layer plays a part as the frame to support the construction of the mucosa and lesion itself. Destruction of the structure of blood vessels can be as easy as that of tent that only needs to untie the ropes of horizontal vessel network and remove the prop of penetrating vessels, although the procedure also can be very difficult when the management is wrong. The precise procedure has been described elsewhere (Figs. 2-6).28-32
THE OTHER DIFFICULT CASES AND THE MANAGEMENT FOR THOSEThe lesions with severe fibrosisLesions with severe fibrosis in the submucosal layer are representative difficult cases of ESD, such as those with ulcer scar and those with recurrence after EMR/ESD. In the management of these cases, the main point is to reach the appropriate depth for sumbucosal dissection that is mentioned above. It is important to start the procedure at an area with little fibrosis where the mucosal flap can be surely formed, even if the tumor free lateral margin may be wider by 1 to 2 cm. The expansion of the resected area seldom influences the procedure time, and the procedure time in the expanded resection can be rather shorter than in the difficult dissection that was started near the ulcer scar. Once the entry into the submuosal layer is carried out, the dissection around the fibrotic area should be possible. The definition of the stream of muscle layer enables the assumption of the appropriate depth for dissection in the fibrotic area by connecting the lines of muscle layer at both sides. Local injection of viscous solution such as hyaluronic acid is also necessary to expand the submucosal layer as much a possible, while local counter-traction can be applied to the space between the muscle layer and the lesion by using the tip of sheath of Flush knife (1 or 1.5 mm) as the divider in cases where local injection is insufficient because of severe fibrosis. In the dissection of a fibrotic area, it is important to proceed by 1 to 2 mm instead of covering a long distance. During the dissection by small distance, it is safe and reliable to approach only to the part that has the little fibrotic area on both sides. This enables new little fibrotic area in the area that has not been dissected, and leads to the assumption of an appropriate line for dissection. In these cases dissection using force coagulation mode is difficult, and change to swift coagulation mode or cut mode will enable sharp cutting (Fig. 7).
The management for difficult cases for approachThe difficult cases for ESD are classified broadly into hard cases for dissection caused by the characteristics of the lesion itself (e.g., cases with easy bleeding and those with severe fibrosis) and hard cases for approach caused by location of the lesion. The management for the former cases is simply the mucosal incision and submucosal dissection at the appropriate depth. The locations in the latter cases are the esophago-gastric junction, pyloric ring of the stomach, ileocecum of the colon, splenic and hepatic flexure of the colon, and anal canal. The situations in those locations are various, and the managements are diverse,36 but the main point is first to incise the most difficult mucosal area for approach (e.g., the duodenal side of the pyloric ring, ileum side of ileocecum, and anal side of anal canal) and dissect the vessel network beneath the edges of incision. Second is to expand the dissection for making the mucosal flap, which allows the entry of scope itself into the submuosal layer. These procedures make the lesion to shift to an easier location by the tension of contralateral mucosa and muscularis mucosae. In cases where the entry into the submucosal layer is difficult, expansion using fishing line is useful.37,38 The appropriate depth of dissection should be commonly given a priority above all in difficult cases for approach.
THE RETRIEVAL OF RESECTED SPECIMEN AND THE MANAGEMENT OF THE ULCER AT THE RESECTION SITEAt the retrieval of the resected specimen, attention must be paid not to damage the specimen. Usually, the specimen is retrieved in a protective manner by grasping the submucosal side of the lesion, often folding the lesion. The grasping of the vessel networks at the submucosal edge of the specimen prevents parts of the specimen from breaking off. For fragile specimens like colorectal lesions, the retrieval net is used. Large specimens that do not fit the net can be sometimes retrieved by pulling out the specimen with a part of the specimen drawn into the sliding overtube. For huge specimens that do not fit the overtube, it is recommended to pack the specimen into a small grip-seal plastic bag and then to retrieve both the specimen and the bag.38,39
Postoperative bleeding is one of the major complications of ESD. It is especially problematic in the stomach and lower rectum. However, few edges of blood vessel are recognized on the artificial ulcer of the resection site when the vessel network is dissected at the appropriate depth. These edges are those of horizontal vessel network and those of vertical penetrating vessels on the artificial ulcer. After the resection, the edges of vessels must be grasped, lifted then coagulated using hemostatic forceps to prevent postoperative bleeding.35 On the other hand, postoperative bleeding seldom occurs in the esophagus and colorectum. Coagulation of the pulsating visible vessels should be minimized to prevent postoperative perforation caused by transmural thermal injury of muscle layer.
THE QUALITY OF THE RESECTED SPECIMEN REQUIRED FROM THE VIEWPOINT FOR THE CRITERIA OF ESDThe characteristics of the resected specimen with controlled appropriate depth of submuosal dissectionThe depth of submucosal dissection mentioned above is the level with little electric resistance, therefore the dissection is smooth and the ulcer at the resection site and the resected specimen are minimally injured by the coagulation. The resected specimen contains thick submucosal layer including the whole vessel network in the submucosal layer (Fig. 6B). This is not only technically useful but also important from the point of view of the quality of the resected specimen.
The required quality of the resected specimenESD is only local resection although it has enabled the endoscopic en bloc resection of lesions with large diameter and ulcer scar. Preoperative diagnosis has limitations, and the criteria for ESD are based on the histopathological diagnosis of the resected specimen. For the histopathological diagnosis, it is necessary to correctly judge the depth of submucosal invasion, histopathological type and pattern at the invasion front, and lymphovascular invasion. Moreover, the vertical margin of specimen must naturally be free of tumor for security of curability. To satisfy these factors, it is necessary to obtain a less physically damaged and thermally denatured resected specimen with sufficiently thick submcosal tissue (ideally the whole layer of submucosa).
Fig. 8 is the case where ESD was performed with a preoperative diagnosis of mucosal cancer. It was a difficult case in the posterior wall of the gastric body, however smooth dissection between the vessel network and muscle layer was possible. The thick submucosal tissue with the whole vessel network can be recognized in the resected specimen. The invasion depth is 260 ┬Ąm in this slice, and the lymphovascular invasion was also detected in other slices. If the dissection was performed in the shallow layer of submucosa, these important findings might have been lost in the specimens and survive in the ulcer at the resection site. Otherwise, these findings can be missed by the thermal denaturation if the vessel network was damaged and repetitive hemostasis was performed. These resections and specimens of low quality might lead to misjudging the case as curatively resected. Cases have been actually reported where recurrences were recognized after the diagnosis of the curative resection. On the contrary, there are cases that are misjudged as being outside the criteria of current guideline. The case in Fig. 9 has the invasive-like area in the submucosa, however it must be judged as a mucosal cancer because the muscularis mucosae covers this area without break. If the dissection is performed in the shallow layer of the submucosa and the muscularis mucosae is damaged, the specimen could be judged as a massively invasive submucosal cancer with positive vertical margin of tumor and the surgical resection might be performed. Misjudgment of the histopathlogical diagnosis for the resected specimen with low quality would unfairly harm the quality of life of the patient by needless total gastrectomy. In fact, correct histopathological diagnosis was possible as presented in the figure, therefore no additional surgical resection was performed and the quality of life has been preserved without recurrence.
No recurrence was observed for over 5 years without additional surgical resection.
Thus the space between the vessel network and the muscle layer can be concluded to be the appropriate depth for dissection.
TREATMENT RESULTS IN OUR UNITWe have already performed 3,740 ESD cases focusing on the appropriate depth for submucosal dissection until May 2012 (esophagus 559 cases; stomach 2,038 cases; and colorectum 1,143 cases). En bloc resection rate was 99.6% for the esophagus, 99.5% for the stomach, and 99.3% for the colorectum; en bloc R0 resection rate was 97.9% for the esophagus, 97.4% for the stomach, and 99.3% for the colorectum. The median procedure time was 56 minutes for the esophagus (range, 15 to 209), 49 minutes for the stomach (range, 8 to 581), and 54 minutes for the colorectum (range, 4 to 622). The perforation rate was 0.9%, 2.1%, and 1.4%, respectively, and these are lower than standard perforation rate (5% to 6%). There was no uncontrollable intraoperative bleeding, and the incidence of postoperative bleeding was 0.4% for the esophagus, 3.2% for the stomach, and 1.2% for the colorectum. No recurrence was observed in the curatively resected cases under the within guideline and expanded criteria40) that were followed without additional surgical resection (median follow-up period, 60.2 months) (Table 2).
CONCLUSIONSThe approach to reach the space between the submucosal vessel network and the muscle layer is necessary for safe and reproducible ESD, especially in difficult cases. These procedures are simultaneously important to obtain a resected specimen of high quality to enable evaluation of the lymphovascular invasion and also the invasion depth and pattern.
THE POSTSCRIPT"Anything essential is invisible to the eyes," says Saint-Exupery in "The Little Prince," whereas everything essential is clearly observed during the procedure of ESD. However, lack of delicate technique and politeness to treat the important object may cause invisibleness of anything essential. Any endo-knives can be used to the preference of the operator, but all operators are requested to perform quality controlled ESD with attention to maintain the appropriate depth for submucosal dissection and to minimize the damage to lesions and muscle layer. ESD can be a superb treatment method that is extremely beneficial for patients when the quality is well secured.
AcknowledgmentsWe wish to thank Dr. Chang-Il Kwon (Digestive Disease Center, Bundang CHA Hospital, Seongnam, Korea) who named our procedure quality controlled ESD for his kind advice in regard to the preparation of this manuscript.
References1. Hirao M, Masuda K, Asanuma T, et al. Endoscopic resection of early gastric cancer and other tumors with local injection of hypertonic saline-epinephrine. Gastrointest Endosc 1988;34:264ŌĆō269. 3391382.
2. Gotoda T, Kondo H, Ono H, et al. A new endoscopic mucosal resection procedure using an insulation-tipped electrosurgical knife for rectal flat lesions: report of two cases. Gastrointest Endosc 1999;50:560ŌĆō563. 10502182.
3. Ono H, Kondo H, Gotoda T, et al. Endoscopic mucosal resection for treatment of early gastric cancer. Gut 2001;48:225ŌĆō229. 11156645.
4. Oyama T, Kikuchi Y. Endoscopic mucosal resection in the upper GI tract-Hook knife EMR method. Minim Invasive Ther Allied Technol 2002;11:291ŌĆō295.
5. Yahagi N, Fujishiro M, Kakushima N, et al. Endoscopic submucosal dissection for early gastric cancer using the tip of an electrosurgical snare (thin type). Dig Endosc 2004;16:34ŌĆō38.
6. R├Čsch T, Sarbia M, Schumacher B, et al. Attempted endoscopic en bloc resection of mucosal and submucosal tumors using insulated-tip knives: a pilot series. Endoscopy 2004;36:788ŌĆō801. 15326574.
7. Neuhaus H, Costamagna G, Devi├©re J, et al. Endoscopic submucosal dissection (ESD) of early neoplastic gastric lesions using a new double-channel endoscope (the "R-scope"). Endoscopy 2006;38:1016ŌĆō1023. 17058167.
8. Kodashima S, Fujishiro M, Yahagi N, Kakushima N, Omata M. Endoscopic submucosal dissection using flexknife. J Clin Gastroenterol 2006;40:378ŌĆō384. 16721217.
9. Yamamoto H, Yahagi N, Oyama T. Mucosectomy in the colon with endoscopic submucosal dissection. Endoscopy 2005;37:764ŌĆō768. 16032498.
10. Oda I, Saito D, Tada M, et al. A multicenter retrospective study of endoscopic resection for early gastric cancer. Gastric Cancer 2006;9:262ŌĆō270. 17235627.
11. Korenaga D, Haraguchi M, Tsujitani S, Okamura T, Tamada R, Sugimachi K. Clinicopathological features of mucosal carcinoma of the stomach with lymph node metastasis in eleven patients. Br J Surg 1986;73:431ŌĆō433. 3719265.
12. Ell C, May A, Gossner L, et al. Endoscopic mucosal resection of early cancer and high-grade dysplasia in Barrett's esophagus. Gastroenterology 2000;118:670ŌĆō677. 10734018.
13. Tanabe S, Koizumi W, Mitomi H, et al. Clinical outcome of endoscopic aspiration mucosectomy for early stage gastric cancer. Gastrointest Endosc 2002;56:708ŌĆō713. 12397280.
14. Oyama T, Tanaka M, Tomori A, et al. Prognosis of endoscopic submucosal dissection for early gastric cancer, results of 3 years or more after treatment. Stomach Intest 2006;41:87ŌĆō90.
15. Onozato Y, Ishihara H, Iizuka H, et al. Endoscopic submucosal dissection for early gastric cancers and large flat adenomas. Endoscopy 2006;38:980ŌĆō986. 17058161.
16. Hirasaki S, Kanzaki H, Matsubara M, et al. Treatment of over 20 mm gastric cancer by endoscopic submucosal dissection using an insulation-tipped diathermic knife. World J Gastroenterol 2007;13:3981ŌĆō3984. 17663514.
17. Oyama T, Tomori A, Hotta K, et al. Endoscopic submucosal dissection of early esophageal cancer. Clin Gastroenterol Hepatol 2005;3(7 Suppl 1):S67ŌĆōS70. 16013002.
18. Fujishiro M, Yahagi N, Kakushima N, et al. Endoscopic submucosal dissection of esophageal squamous cell neoplasms. Clin Gastroenterol Hepatol 2006;4:688ŌĆō694. 16713746.
19. Fujishiro M, Yahagi N, Kakushima N, et al. Outcomes of endoscopic submucosal dissection for colorectal epithelial neoplasms in 200 consecutive cases. Clin Gastroenterol Hepatol 2007;5:678ŌĆō683. 17466600.
20. Tamegai Y, Saito Y, Masaki N, et al. Endoscopic submucosal dissection: a safe technique for colorectal tumors. Endoscopy 2007;39:418ŌĆō422. 17516348.
21. Cao Y, Liao C, Tan A, Gao Y, Mo Z, Gao F. Meta-analysis of endoscopic submucosal dissection versus endoscopic mucosal resection for tumors of the gastrointestinal tract. Endoscopy 2009;41:751ŌĆō757. 19693750.
22. Oda I, Gotoda T, Hamanaka H, et al. Endoscopic submucosal dissection for early gastric cancer: technical feasibility, operation time and complications from a large consecutive series. Dig Endosc 2005;17:54ŌĆō58.
23. Kakushima N, Fujishiro M, Kodashima S, Muraki Y, Tateishi A, Omata M. A learning curve for endoscopic submucosal dissection of gastric epithelial neoplasms. Endoscopy 2006;38:991ŌĆō995. 17058163.
24. Saito Y, Uraoka T, Matsuda T, et al. Endoscopic treatment of large superficial colorectal tumors: a case series of 200 endoscopic submucosal dissections (with video). Gastrointest Endosc 2007;66:966ŌĆō973. 17524403.
25. Tanaka S, Oka S, Kaneko I, et al. Endoscopic submucosal dissection for colorectal neoplasia: possibility of standardization. Gastrointest Endosc 2007;66:100ŌĆō107. 17591481.
26. Participants in the Paris Workshop. The Paris endoscopic classification of superficial neoplastic lesions: esophagus, stomach, and colon: November 30 to December 1, 2002. Gastrointest Endosc 2003;58(6 Suppl):S3ŌĆōS43. 14652541.
27. Kitajima K, Fujimori T, Fujii S, et al. Correlations between lymph node metastasis and depth of submucosal invasion in submucosal invasive colorectal carcinoma: a Japanese collaborative study. J Gastroenterol 2004;39:534ŌĆō543. 15235870.
28. Toyonaga T, Nishino E, Hirooka T. Invention of water jet short needle knives (Flush knife) for endoscopic submucosal dissection. Endosc Dig 2005;17:2167ŌĆō2174.
29. Toyonaga T, Nishino E, Hirooka T, et al. Use of short needle knife for esophageal endoscopic submucosal dissection. Dig Endosc 2005;17:246ŌĆō252.
30. Toyonaga T, Nishino E, Hirooka T, Ueda C, Noda K. Intraoperative bleeding in endoscopic submucosal dissection in the stomach and strategy for prevention and treatment. Dig Endosc 2006;18(Suppl 1):S123ŌĆōS127.
31. Toyonaga T, Man-i M, Morita Y, et al. Esophageal endoscopic submucosal dissection using the FlushKnife-efficiency in creating the submucosal groove and tunnel method with additional injection from the knife. Stomach Intest 2009;44:378ŌĆō383.
32. Toyonaga T, Man-i M, Fujita T, et al. Endoscopic submucosal dissection using the Flush knife and the Flush knife BT. Tech Gastrointest Endosc 2011;13:84ŌĆō90.
33. Toyanaga T, Man IM, Ivanov D, et al. The results and limitations of endoscopic submucosal dissection for colorectal tumors. Acta Chir Iugosl 2008;55:17ŌĆō23. 19069688.
34. Toyonaga T, Inokuchi H, Man-i M, et al. Endoscopic submucosal dissection using water jet short needle knives (Flushknife) for the treatment of gastrointestinal epithelial neoplasms. Acta Endoscopica 2007;37:645ŌĆō655.
35. Toyonaga T, Man IM, Fujita T, et al. The performance of a novel ball-tipped Flush knife for endoscopic submucosal dissection: a case-control study. Aliment Pharmacol Ther 2010;32:908ŌĆō915. 20839389.
36. Toyonaga T. ESD Atlas. 2006. Tokyo: Kanehara Shuppan.
37. Tsao SK, Toyonaga T, Morita Y, Fujita T, Hayakumo T, Azuma T. Modified fishing-line traction system in endoscopic submucosal dissection of large esophageal tumors. Endoscopy 2011;43(Suppl 2 UCTN):E119. 21425004.
38. Toyonaga T, Man-i M, Fujita T, et al. Retrospective study of technical aspects and complications of endoscopic submucosal dissection for laterally spreading tumors of the colorectum. Endoscopy 2010;42:714ŌĆō722. 20806155.
39. Tanaka S, Toyonaga T, East J, et al. Endoscopic retrieval method using a small grip-seal plastic bag for large colorectal resection specimens after endoscopic submucosal dissection. Endoscopy 2010;42(Suppl 2):E186ŌĆōE187. 20680918.
40. Toyonaga T, Man-I M, East JE, et al. 1,635 Endoscopic submucosal dissection cases in the esophagus, stomach, and colorectum: complication rates and long-term outcomes. Surg Endosc 2012 10 06 DOI: 10.1007/s00464-012-2555-2.
Fig.┬Ā1(A-C) The worst postulated sequence of possible events of endoscopic submucosal dissection. The bleeding occured just after the beginning of incision and dissection. The procedure was finally finished with frequent hemostasis with difficulty. The edge of incision was burned to be tough, and the entry of the devices into the submucosal layer was impossible beneath the incised mucosa, like a shell closing its husk. The entry into the submucosal layer was carried out from other mucosa, however the massive bleeding occurred during the dissection. 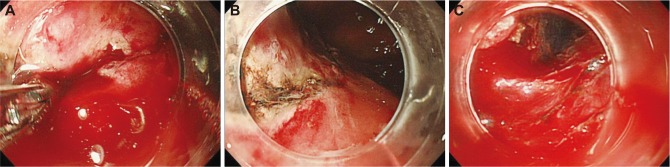
Fig.┬Ā2The structure of vessels in the gastrointestinal luminal wall. (A) The vessel network in the submucosal layer. The shallow mucosal incision just below the muscularis mucosae. Smooth incision with less hemorrhage is enabled, and the horizontal vessel network is seen through at the middle level of the submucosal layer. (B) The large vessels in the gastrointestinal luminal wall penetrate the muscle layer vertically and then inflow horizontally at the level of middle submucosal layer forming the ramified vascular network. In the area with high density of vessels, the faciae-like layer is formed with the ramified vascular network and the perivascular fibrotic tissue, and the layer containing fewer vessels and fibrotic tissue exists below the faciae and just above the muscular propria. Smooth dissection with less hemorrhage is enabled by maintaining the appropriate depth of dissection at the layer with fewer vessels and fibrotic tissue and by coagulating the penetrating vessels before dissection. 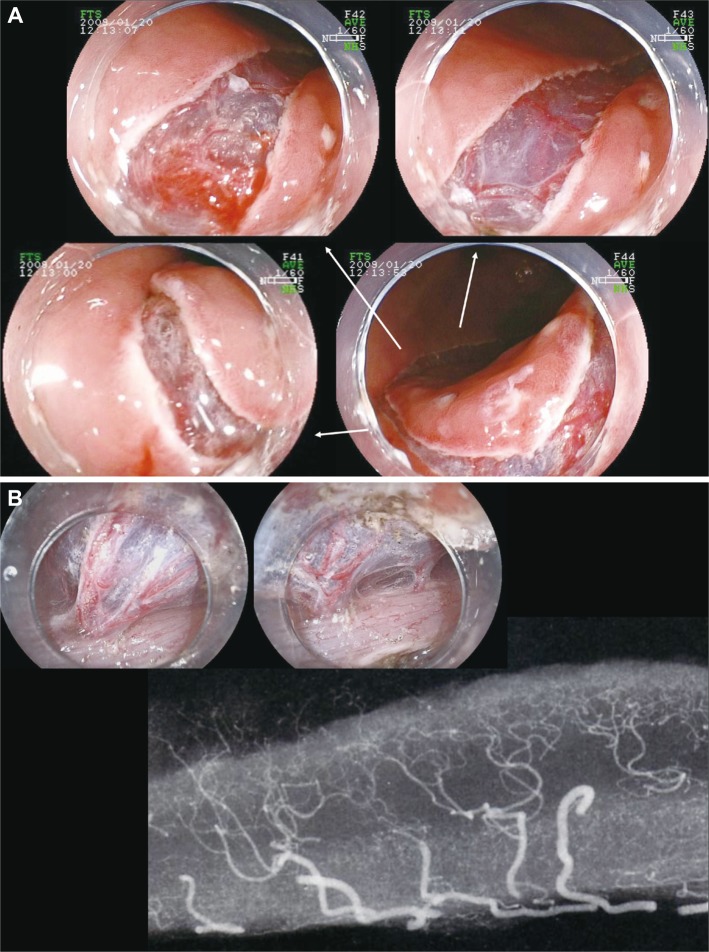
Fig.┬Ā3(A) An early stage gastric cancer in the posterior wall of body. (Ba) The horizontal vessel network in the submucosal layer is partially transected to reach the appropriate depth of dissection. The large vessels vertically penetrate the muscle layer and ramify horizontally at the middle level of the submucosal layer. (Bb) The ramified vessel network in the submucosal layer also connects the edges of incision. The certain dissection of this network allows the entry into the appropriate depth of submucosal dissection by broadening the groove made by the mucosal incision. (C) Once the appropriate depth of submucosal dissection has been obtained, the smooth dissection is enabled with first recognizing and properly dissecting the occasional penetrating vessels. 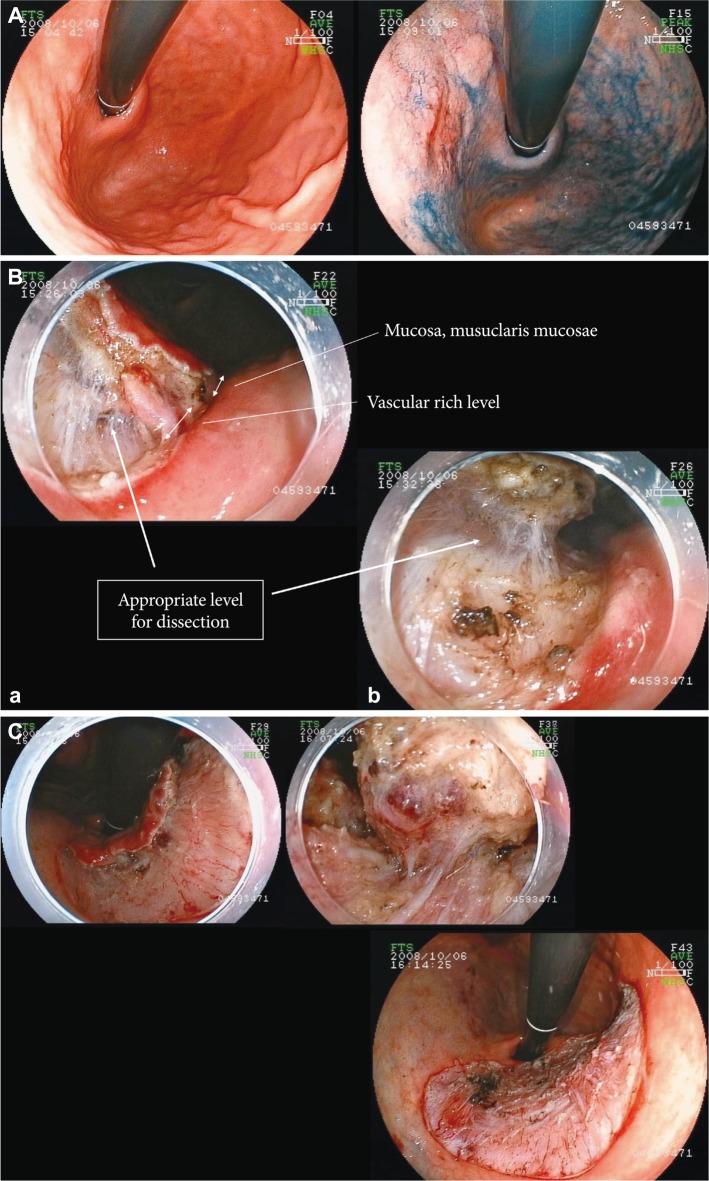
Fig.┬Ā4The difference of the density of vessels. (A) The extremely characteristic muscle layers named the oblique muscle layers are symmetrically seen in the anterior/posterior regions of the gastric body. The muscle layer is circularly absent at the inlet of the large vessel. (B) The density and thickness of the vessels in the gastric antrum overwhelmingly differs from those in the gastric body. (Ba) In the antrum, the density of vessel in the submucosal layer is low, and the fibrosis is also minimal, and these allow easy mucosal incision and submucosal dissection. (Bb) In the lesser curvature of the gastric body where the oblique muscle layers exist, as the blood vessels do not diverge frequently and the density of blood vessels are low as in the gastric antrum, the procedure is rather easy if the large penetrating vessels are not hurt by mistake. (Bc) On the other hand, in the anterior/posterior walls of the gastric body, where the oblique muscle layers exist, the greater curvature of the gastric body and lower rectum, the density of blood vessels is high and the diverged vessel network is inevitably hurt, if careful attention is not paid to the depth of mucosal incision and submucosal dissection. 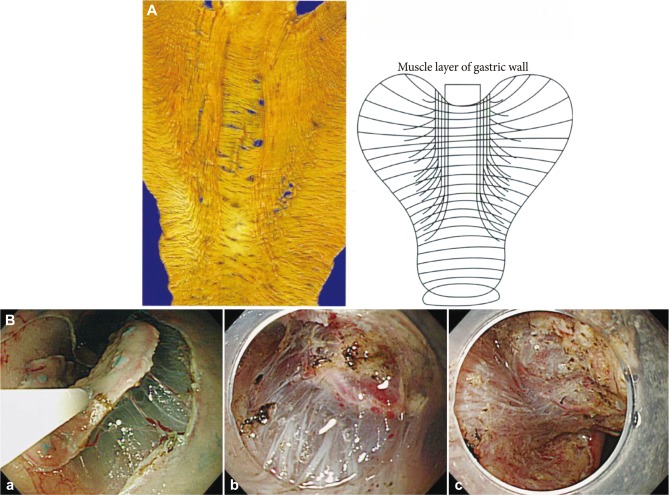
Fig.┬Ā5Laterally spreading tumor: granular type in rectum. (A) Subtotal circumferential lesion. The anal side of lesion reached to the anal canal. The rectum, especially lower rectum and anal canal have largest blood vessels and also highest vessel density in the rectum. The shallow mucosal incision from the anal side was performed not to damage the vessels, and then the vessel network beneath the edges of incision was transected to reach the appropriate depth of dissection. (B) Bleeding does not occur if the dissection was performed beneath the horizontal vessel network while averting the penetrating vessels. The structure of blood vessels in the rectum was clearly recognized. The muscle layer is absent at the inlet of the large vessel in the shape of a wedge. 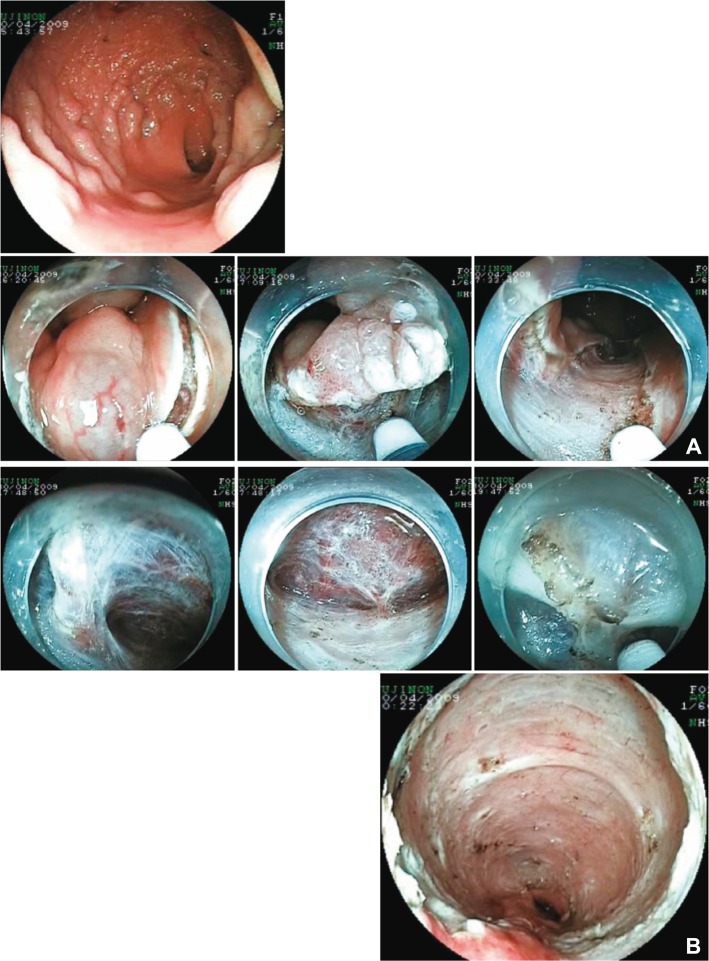
Fig.┬Ā6Quality controlled endoscopic submucosal dissection (ESD). (A) The technical tip to maintain the appropriate depth of submucosal dissection (upper). A shallow mucosal incision is performed not to cause bleeding. The horizontal vessel network is disclosed by dissecting the space between the vessels that can be seen through in the submucosal layer. After precoagulation and dissection of the vessel network to scoop up from the bottom of the vessel into the lumen, the edge of mucosal incision easily expands and the transparent hood itself can get into the submucosal layer and then a mucosal flap is formed. The technical tip to maintain the appropriate depth of dissection (lower). Once the blood vessels are damaged by mistake, the field of vision is lost and subsequent procedure becomes difficult, even after the appropriate depth of dissection has been obtained. The measure is to dissect with occasional local injection beneath the vessel network while maintaining the field of vision by the transparent hood. Especially, it is always necessary to distinguish the recognized vessels whether these are horizontal small branches of the vessel network or vertical large trunks of the penetrating vessels. (B) The resected specimen obtained by quality controlled ESD. The resected specimen contains thick submucosal layer including the whole vessel network in the submucosal layer. The appropriate depth of submucosal layer has most sparse and little electric resistance and the resected specimens are minimally injured by the coagulation. 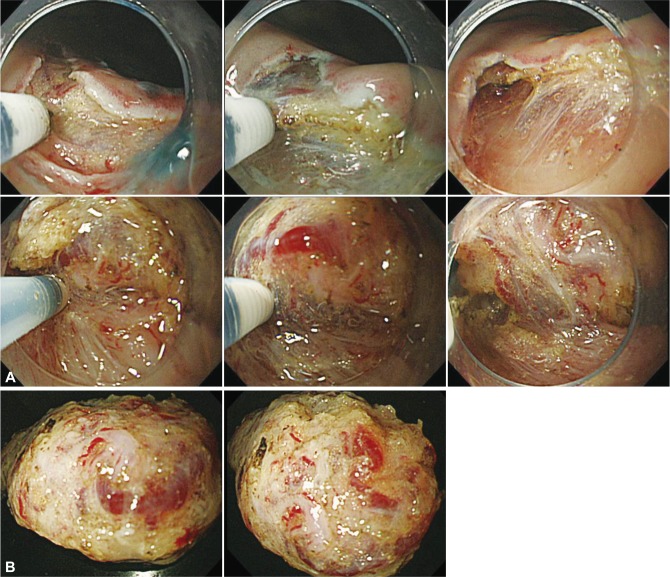
Fig.┬Ā7Endoscopic submucosal dissection (ESD) of the lesions with severe fibrosis. (A) Laterally spreading tumor: non granular type in the colon. A mucosal flap was formed by dissection of the area with little fibrosis as much as possible after reaching the appropriate depth of submucosal dissection. The transparent hood could get into the submucosal layer despite the fibrosis because the appropriate depth of dissection has already been obtained. The fibrotic area can be safely and certainly dissected by dividing the muscle layer and lesions using the sheath of Flush knife and also connecting the lines of muscle layer at both sides. (B) The view after dissection of the fibrotic area and the resected specimen. The lesion was accompanied with severe fibrosis; however, the high-quality resected specimen was obtained with the entire vessel network. In ESD of lesion with severe fibrosis, it is important first to reach the appropriate depth of submucosal dissection (H&E stain, ├Ś12.5). 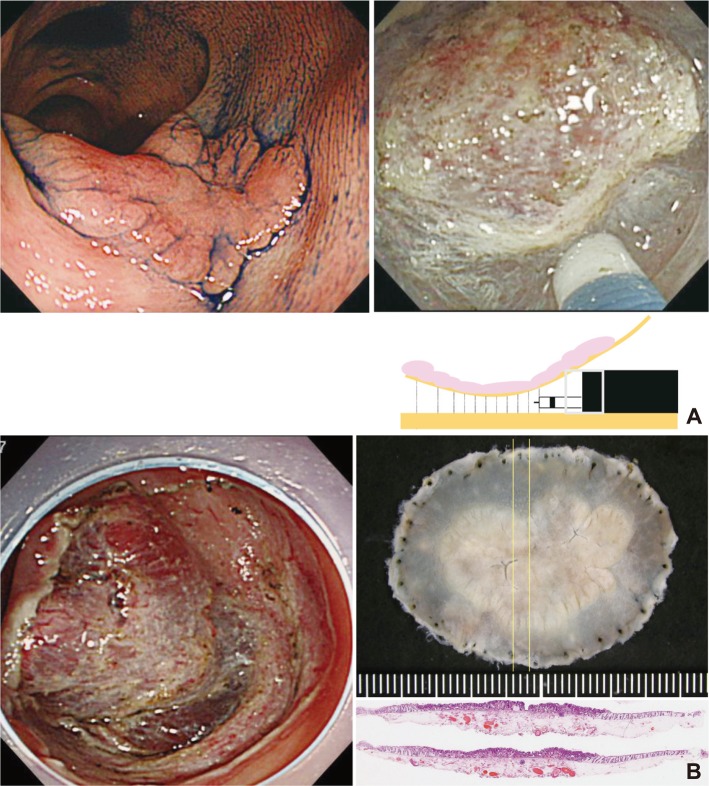
Fig.┬Ā8The resected specimen of a case which was revealed to be outside the criteria. 0-IIc lesion in the posterior wall of the gastric body. It was diagnosed as a mucosal cancer and endoscopic submucosal dissection was performed. The resected specimen was obtained with thick submucosal layer containing all of vessel network. The submucosal invasion to the depth of 260 ┬Ąm and lymphovascular invasion was recognized (H&E stain, ├Ś40). If the dissection in the shallow submucosal layer was performed, these important findings might not be contained in the resected specimen and remain in the artificial ulcer of the resection site. If the vessel network was damaged and repetitive hemostasis was performed, these findings could not be recognized. These low-quality resection and specimen might lead to misjudge the case as curatively resected. M, mucosal; SM, submucosal; tub., tubular adenocarcinoma; ly, lymphatic invasion; LM, lateral margin; VM, vertical margin; EC, curativity C. 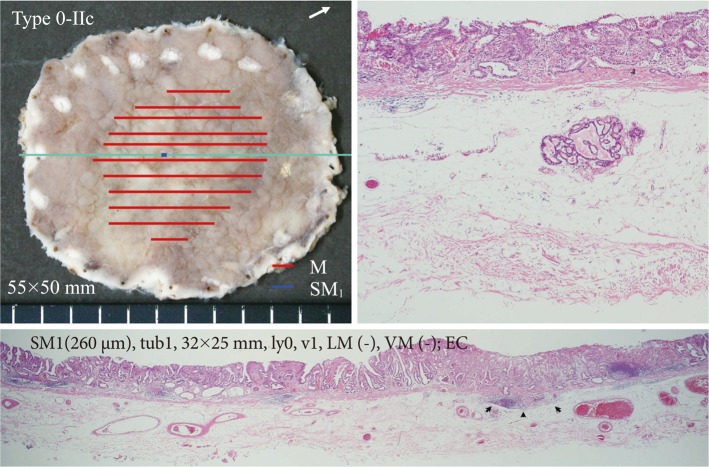
Fig.┬Ā9The resected specimen of a case which was confirmed to be within the criteria. The tumor invaded into the deep layer of the submucosa, but the muscularis mucosae covered this area without break (H&E stain, ├Ś40). This was diagnosed as mucosal cancer. If the dissection was performed in the shallow layer of the submucosa and the muscularis mucosae was damaged, the specimen could be judged as massively invasive submucosal cancer with positive vertical margin of tumor. M, mucosal; tub., tubular adenocarcinoma; ly, lymphatic invasion; LM, lateral margin; VM, vertical margin; EA, curativity A. 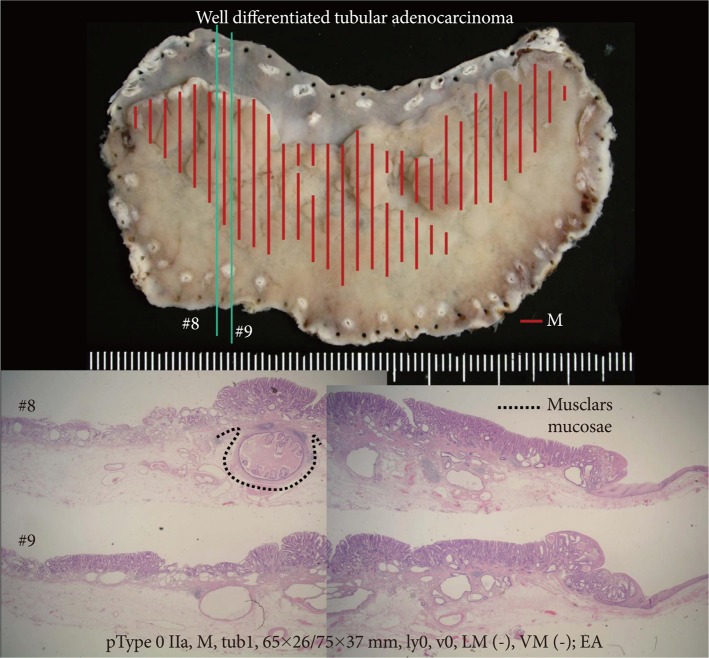
|
|
|||||||||||||||||||||||||||||||||||||||||
