INTRODUCTION
The use of metal stents in the gastrointestinal tract is one of the most influential developments in the interventional endoscopy. The first clinical application of a metal stent in a non-vascular organ was on the tracheobronchial tree by Wallace et al. [1]. Metal stent technology has evolved rapidly over the past decade. Recent technological developments in interventional endoscopic ultrasonography (EUS) and metal stents have facilitated EUS-guided procedures using metal stents in various gastrointestinal conditions.
Using linear-array echoendoscopes, a needle can be advanced into the biliary or pancreatic duct under real-time ultrasonographic guidance without the risk of injury to the intervening vessel. Because the common bile duct and the pancreatic duct are near the transducer of the EUS probe, both can be visualized without difficulty, and real-time puncture can be done easily, thus providing ductal drainage using endoscopic retrograde cholangiopancreatography (ERCP)-based techniques [2,3]. Over the past decades, the availability of the linear-array echoendoscope has led to the development of multiple EUS-related endoscopic techniques, such as pancreatic fluid collection (PFC) drainage, pancreaticogastrostomy, EUS-guided antegrade cholangiography and hepaticogastrostomy (HGS), and choledochogastrostomy [4]. In the early 2000s, the availability of a large-channel echoendoscope induced the first reported case of EUS-guided transgastric (EUS-HGS) or transduodenal (choledochoduodenostomy, EUS-CDS) biliary drainage using stents by Giovannini et al. [5,6]. EUS has since evolved continuously from a only diagnostic imaging method to an invasive therapeutic procedure. We will review new EUS-guided metal stents and future developments in gastrointestinal diseases and other fields.
LITERATURE REVIEW METHODOLOGY
On November 15, 2015, a PubMed search using the EUS in combination with words related to metal stents, such as ŌĆ£metal stentŌĆØ and ŌĆ£therapeutic EUS,ŌĆØ was done. Next, the inclusion criteria were (1) original research articles (randomized controlled trials, prospective and retrospective studies), systemic reviews, and case series; (2) case number Ōēź20; and (3) English-language publications (Fig. 1).
The publications and their references were reviewed manually. References to proper studies and review articles were searched manually to survey additional related studies. The bibliographies of the review articles were scrutinized to identify other references that might have been missed in the initial search. Finally, the two authors of this article jointly reviewed each published paper, and all relevant information was extracted.
PANCREATIC FLUID COLLECTION
PFCs include peri-PFCs, pancreatic pseudocysts, acute necrotic collections, and walled-off pancreatic necrosis [7,8]. PFCs generally require drainage when they cause symptoms such as pain, gastric outlet obstruction (GOO), and/or bile duct obstruction, or if they are infected [9]. The management of PFCs has traditionally been surgical, but surgery may be associated with relatively higher rates of complications and mortality [10]. With more recent technological advances and experience, endoscopic drainage is widely accepted and has replaced surgery as the first-line therapy for PFC drainage, as it is less invasive and has a shorter recovery time, lower cost, and lower complication rates [11].
EUS guided-endoscopic transmural drainage of PFCs with plastic or metal stents has become a widely accepted treatment modality. Previous studies have reported success rates comparable to those of percutaneous and surgical interventions, with significant advantages in terms of invasiveness, morbidity, length of hospital stay, and cost [12]. The procedure is preferably performed under EUS guidance, which allows access to non-bulging lesions, avoidance of major vessels, and accurate assessment of PFC content, which is critical for the selection of the number and type of stent to be placed. The clinical success rate of EUS-guided PFCs drainage is directly related to the necrotic content of PFCs, which increases the risk of occlusion of the stents typically used for this type of drainage procedure. Historically, double-pigtail plastic stents have been the mainstay of therapy. However, because of their small caliber, plastic stents can become occluded with the development of secondary infection; thus, increasing the need for re-intervention and surgical treatment when endoscopic re-treatment fails. Moreover, if a necrosectomy is required following drainage by plastic stents, the endoscopist must first remove the stents and then drive the scope through the drainage tract to extract the necrotic material, which often requires multiple tract dilations and scope insertions into the PFC and increases the risk of procedural morbidity.
To overcome these limitations, fully covered self-expanding metal stents (FCSEMSs) and specially designed lumen-apposing metal stents (LAMSs) in which both ends are flared have been proposed, with the rationale of providing a large opening that would theoretically allow for longer patency, reduce rates of stent occlusion and decrease the probability of secondary infections, and provide a lasting approach route to perform multiple tissue sections via necrosectomy [7,9,13,14]. In a retrospective cohort study including 230 patients with PFCs, especially pancreatic pseudocysts, EUS-guided drainage using FCSEMSs improved clinical outcomes (complete resolution of pseudocyst, 98% vs. 89%, p=0.01) and reduced adverse event rates (16% vs. 31%, p=0.006) compared with plastic stents [15]. A prospective comparative study demonstrated that EUS-guided PFC drainage using a FCSEMS was comparable to that using plastic stents regarding technical feasibility, efficacy, and safety. The median procedure time using a FCSEMS was significantly shorter than that using plastic stents (15.0 minutes vs. 29.5 minutes, p<0.01) [16].
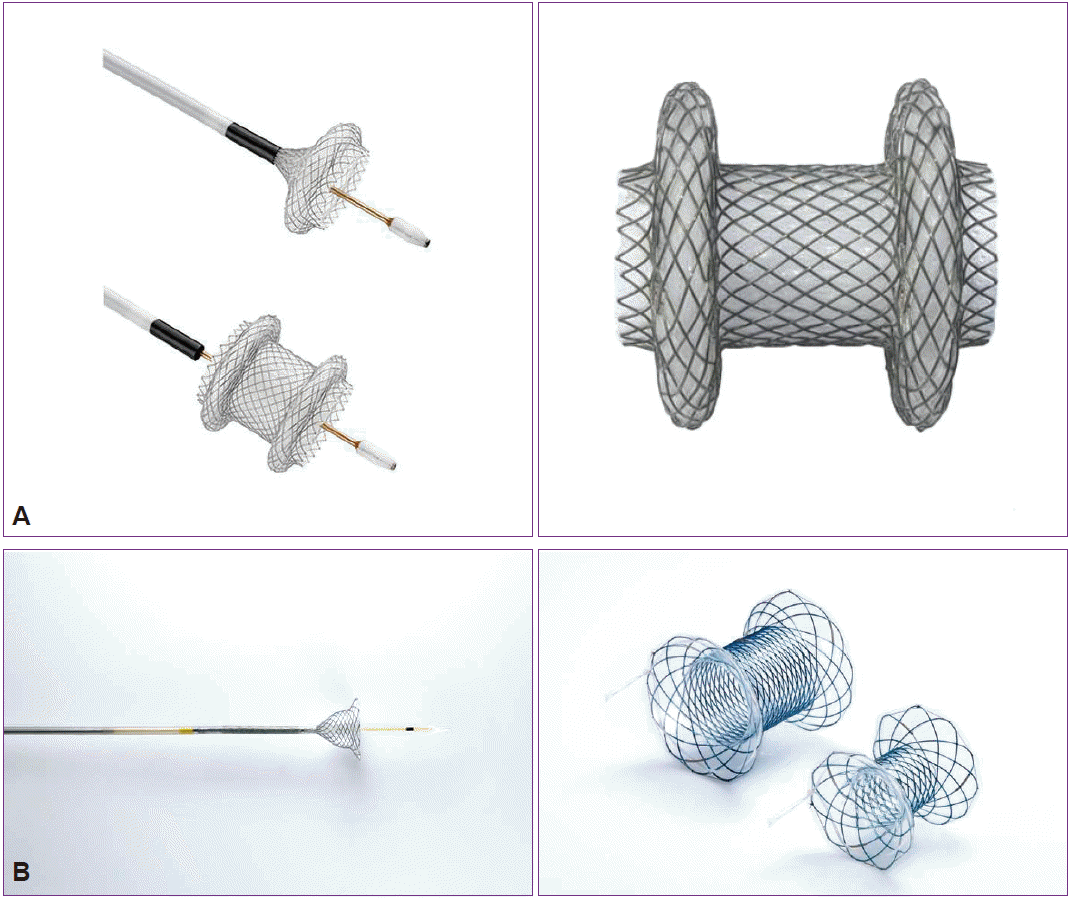
Recently, endoscopists have seen the development and commercial release of specially designed, fully covered, transluminal self-expanding metal stents (SEMSs), such as the AXIOS (Boston Scientific, Natick, MA, USA) and Nagi (Taewoong Medical, Goyang, Korea) stents, which are designed with wide flanges on both ends to prevent migration (Fig. 2). These devices have a wide diameter (10 to 16 mm) and short length, and to some extent, they can actively hold a PFC in the lumen. Endoscopic necrosectomy can be performed directly through these devices owing to their wider diameter. Large cohort studies have demonstrated high technical success rates (91% to 98%) and clinical success rates, defined as resolution of clinical symptoms with a decrease in PFC size to Ōēż2 cm on imaging (81% to 100%) [7,17,18].
Few data are available regarding the proper period for stent removal. One prospective, single-center study showed the safety and efficacy of short-duration (3 weeks) FCSEMS placement for symptomatic pseudocysts, along with selective pancreatic ductal stenting in patients with persistent ductal leak. During a median follow-up of 306 days, two recurrences (4.7%) were detected in 42 patients [19]. This study demonstrated that short-term placement of FCSEMSs with pancreatic ductal stenting for the treatment of pseudocysts is safe and effective.
FCSEMSs have been used with the hope that a large luminal diameter would facilitate more effective and lasting drainage. However, unfortunately, SEMSs may migrate and have been reported to cause mucosal injury and bleeding from erosion of the opposite wall of the cavity once the PFC is resolved. A recent study showed a 26% incidence of late adverse events after FCSEMS placement for PFCs [20]. A systematic review including 17 studies and 881 patients who underwent endoscopic treatment of PFCs using plastic versus biliary FCSEMSs did not support routine deployment of metal stents for drainage of PFCs, reporting similar pooled success rates for metal stents (81.9%) and plastic stents (80.7%) and higher pooled complication rates (such as bleeding, secondary infection and stent migration) with metal stents (23.3%) compared with plastic stents (16.1%) [21].
Several novel LAMSs specially designed for transmural drainage allow single-step deployment and effective drainage of cystic fluid with minimal stent migration (Table 1) [7,13,14,17,18,22-24]. The AXIOS and Nagi stents have high reported treatment success rates of 93% to 100%, but these results are limited to small studies of 10 to 33 patients. Further randomized trials are needed to justify the use of metal stents for PFC drainage.
Despite advances in metal stent development and the theoretical advantages of metal stents over plastic stents, there is no universal agreement as to which type of stent should be used for drainage of PFCs. Based on the current literature, one stent type cannot be clearly recommended over other types for EUS-guided drainage of PFCs. Endoscopists should choose a stent type they are comfortable using and one that they feel is most appropriate for each individual case. The AXIOS and Nagi stents may be preferable when necrosectomy is required, because the endoscopist can drive the scope directly through the stent and into the PFC for removal of solid necrotic material.
PANCREATIC DUCT DRAINAGE
The development of interventional EUS has provided an alternative means of relieving pancreatic duct obstruction that is not possible by ERCP through transpapillary access to the pancreatic duct, especially in pancreaticojejunal stenosis or pancreaticogastric anastomosis after Whipple resection, which can result in recurrent acute pancreatitis, main pancreatic duct (MPD) stenosis due to chronic pancreatitis, or post-pancreatic damage after failure of ERCP [10]. EUS-guided transmural stenting using plastic stents was performed in cases in which advancing a guidewire across the anastomosis site was impossible, when the major papilla was under complete pancreatic obstruction, or when the MPD had a tortuous configuration [25]. However, rates of complications, including bleeding, fever, perforation, and hematoma, are typically higher for transmural stenting than for simple rendezvous placement [26]. FCSEMSs have not yet been used for EUS pancreatic duct drainage with transmural stenting because of concerns regarding stent migration and a cross-stream blockage of the MPD that covers the membrane of the FCSEMS [25]. Recently, Oh et al. [27] showed that EUS pancreatic duct drainage using a FCSEMS may be technically feasible and relatively safe without adverse events related to FCSEMSs, including stent migration, clogging, and stent-induced ductal stricture. In the future, FCSEMSs should be further modified with an anti-migration structure and appropriate diameter to decrease the risk of branch duct blockage, and a well-designed randomized controlled trial is also needed.
BILE DUCT DRAINAGE
In cases in which papillotomy and bile duct cannulation are not successful, either because of an altered anatomy by previous gastrointestinal surgery or because of a tight tumor stenosis, surgery and/or percutaneous transhepatic biliary drainage (PTBD), which have higher morbidity and mortality rates than those of ERCP, have been suggested as alternative methods [28,29]. EUS-guided biliary drainage (EUS-BD) is considered an effective salvage procedure for failed ERCP in patients with unresectable malignant biliary obstruction. In patients with malignant distal common bile duct obstruction requiring SEMS placement, the short-term composite success defined as the ability to complete the intended therapeutic procedure in a single session and resulting in a greater than 50% decrease in bilirubin over 2 weeks using EUS-BD was reported to be comparable to that of ERCP in a retrospective analysis [30].
In the early 2000s, a large-channel echoendoscope made possible the first reported case of EUS-BD including transgastric or transduodenal biliary drainage, which had been initially described for palliative management of malignant biliary obstruction [5,6,31]. The different access routes (transhepatic or extrahepatic), directions of stent insertion (anterograde or retrograde), or drainage routes (transluminal or transpapillary) have been controversial, with varying success rates and complication rates, for EUS-BD [32]. Although no randomized trial has directly compared the rendezvous and transluminal techniques, both techniques were equally effective and safe according to a small retrospective study, and the authors concluded that the transluminal approach is a reasonable approach secondary to rendezvous EUS-BD, because cumbersome wire manipulations are not needed [33].
A report by a single endoscopist that included 101 patients showed that the risk of procedure-related death decreases with the learning curve, even if EUS-BD is an efficient technique [34]. In connection with this, a newly designed stent introducer having a fine gauge metal tip and a 7 F shaft diameter has been evaluated to enable one-step stent placement without a further fistula dilation step, potentially decreasing the risk of adverse events by immediately sealing the transmural fistula [25].
There are two main approach routes in EUS-BD in terms of the organ on which a non-anatomic fistula is connected to the biliary tree: EUS-CDS and EUS-HGS. The EUS-HGS and EUS-CDS techniques exhibited similar efficacy and safety in a single-center randomized trial [35]. EUS-CDS has several potential advantages compared with endoscopic transpapillary stenting (ETS), including a lower risk of post-procedural pancreatitis, lower risk of tumor ingrowth and overgrowth, and higher technical success rate [35]. A comparative cohort study that evaluated the clinical efficacy and safety of EUS-CDS and ETS as first-line treatments for malignant distal biliary obstruction showed that EUS-CDS was associated with short procedure times and no risk of pancreatitis [35].
PTBD is the most commonly used alternative drainage method for intrahepatic ducts but has a relatively high rate of complications and is frequently associated with patient discomfort related to the external drainage [36]. The advantage of EUS-HGS compared with PTBD is the lower risk of bleeding, through the use of color Doppler, which can prevent puncturing of intervening vessels [3]. However, bile leakage is a potential significant adverse event. In a multicenter retrospective analysis, EUS-HGS was related to complications including cholangitis, perforation, bile leakage, and bleeding (p=0.031) [32]. The left hepatic ducts are easily visualized by EUS; thus, a transgastric approach to the left biliary system was initially introduced, and hepatic hilar obstruction and isolated right intrahepatic duct obstruction are not normal indications for EUS-HGS [37]. However, recently, sequential deployment of two stacked stents was used in isolated right intrahepatic duct obstruction to prevent bile leakage from the fistula and bile duct branch occlusion using FCSEMs. This method is known as the locking-stent method and is performed using an uncovered stent initially deployed from the upper common bile duct to B, and then a bare-ended, fully covered stent is inserted into the first stent from B3 to the stomach [38]. For standardization of EUS-HGS in cases of failed ERCP, the development of a standard protocol for endoscopic procedures and a more specific and dedicated device for effective and safe deployment of metal stents without complications is required [39].
As FCSEMSs offer prolonged stent patency (compared with plastic stents) and easy stent revision without increasing procedural complications such as bile leakage (compared with plastic stents or SEMSs), EUS-BD using FCSEMSs was suggested as the ideal option for failed ERCP cases [40,41]. In addition, EUS-BD using the FCSEMS procedure provides a biliary transmural drainage route away from a duodenal SEMS and thus may be expected to prolong the time to dysfunction of a biliary stent compared with transpapillary drainage in cases with an indwelling duodenal SEMS [42]. FCSEMSs appear to be a better option for EUS-BD because with full expansion, it effectively seals the puncture tract; thus, preventing leakage, and the larger diameter provides better long-term patency. Furthermore, in cases of stent occlusion, revision was more manageable compared with plastic stents, as a new stent can be placed into the occluded metal stent [10].
GALLBLADDER DRAINAGE
Laparoscopic cholecystectomy is the standard approach used for patients with acute cholecystitis [43]. However, some patients are improper for cholecystectomy because of advanced age, underlying comorbidities, or malignancies. In these cases, percutaneous transhepatic gallbladder drainage (PTGBD) is the treatment of choice, and shows clinical success rates of 56% to 100% [44]. Nevertheless, the percutaneous approach has many weak points, including bleeding, pneumoperitoneum, bile leakage, and catheter dislodgement; additionally, long-term percutaneous drainage leads to discomfort in patients. Furthermore, high recurrence rates of cholecystitis of up to 41% have been reported after removal of the drainage catheter [45,46].
Endoscopic methods for gallbladder drainage (GBD) include the transpapillary approach or EUS-guided transmural GBD. Jang et al. [47] showed that EUS-GBD is comparable with PTGBD in terms of technical feasibility and clinical efficacy for the treatment of acute cholecystitis in high-risk surgical patients. However, to date, there are a few data on the safety and feasibility of EUS-guided transmural GBD, because previous tubular-shaped stents have risks, such as bile leakage or migration. Furthermore, the slow flow of bile and the small caliber of plastic stents can result in early malfunction and clogging. Thus, a modified FCSEMS, with enlarged flares (22 mm external diameter) and stents angulated to 90┬░ to prevent migration after placement, was suggested to create a safe fistula tract connecting the bowel and gallbladder without intraperitoneal bile leakage [37]. Recently, specifically designed LAMSs have been developed for transenteric drainage and successfully tested in animal models [39]. In a small case series, EUS-guided cholecystoenterostomy using a LAMS was found to facilitate internal GBD in non-surgical candidates who have a percutaneous cholecystostomy catheter [48]. However, reports on the use of a LAMS for GBD are limited to small case series without long-term follow-up [38]. Recently, in a multicenter prospective study, EUS-GBD using a newly developed LAMS device showed promising results (technical success rate, 90%; clinical success rate, 96%; stent- or procedure-related mortality rate, 7%) in high-risk surgical patients with acute cholecystitis [49].
FUTURE DEVELOPMENT FOR GASTROENTEROSTOMY
Surgical gastroenterostomy has been the standard palliative therapy for malignant GOO due to stomach, duodenum, or pancreatic cancer. Endoscopic placement of an enteric SEMS across the malignant stricture is an alternative treatment option. This procedure leads to recovery of oral intake in ~90% of patients; however, it may be complicated by recurrent obstruction caused by either stent migration or tumor infiltration [50]. An alternative approach is the creation of EUS-guided endoscopic gastroenterostomy using metal stents. Endoscopic gastrojejunostomy (GJS) appears to be ideal for malignant GOO because of the short length of anastomosis in surgical GJS and the less invasive nature of endoscopic metal stent placement. Endoscopic GJS is divided into two approaches, GJS using flexible forward-view endoscopy and that using an echoendoscope [51].
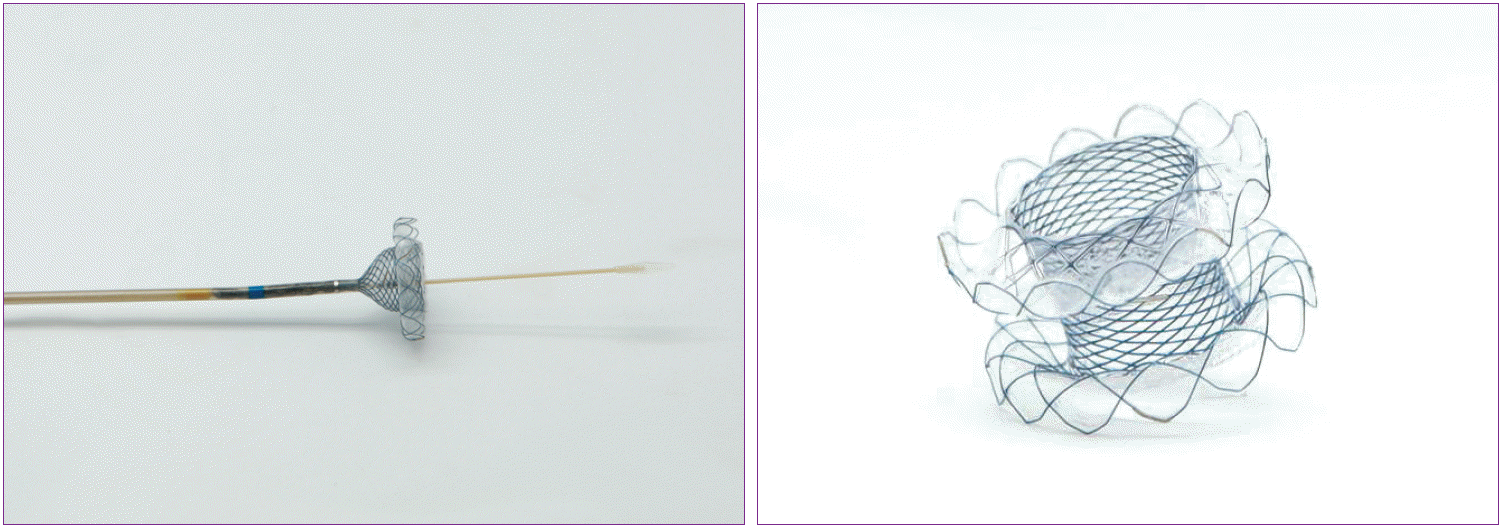
LAMSs such as the Spaxus (Taewoong Medical) or AXIOS stent have been developed, and EUS has been used to guide placement of LAMSs to create gastrogastric fistulas (Fig. 3). Itoi et al. [52] showed that creation of an EUS-guided GJS using a novel enteric balloon and metal stent was promising as a minimally invasive treatment in an animal model. This technique involves utilizing a specially created double-balloon enteric tube (Tokyo Medical University type; Create Medic, Yokohama, Japan) to stabilize the small bowel adjacent to the stomach in the area of the puncture and LAMS placement. A prospective study was performed on EUS-guided double-balloon-occluded GJS bypass using LAMSs in 20 patients in 2015. The technical success rate was 90% (18/20) [51,53].
We hope that in the near future many patients suffering from malignant GOO can benefit from safe endoscopic gastroenterostomy using dedicated metal stents, which enable both secure anastomosis and passage of food. In addition, the prevalence of obesity and diabetes has exponentially increased globally, and conventional bariatric surgery is currently regarded as the only treatment that typically results in large, sustained weight losses and diabetes control for patients intractable to medical treatments. However, bariatric surgery also carries perioperative risks. Therefore there is a great need for minimally invasive weight-loss procedures in treatment sequence. In this context, we believe that the endoscopic bypass procedure using a simple anastomosis method with a LAMS has the potential to be a minimal invasive treatment strategy for metabolic diseases, including obesity and type 2 diabetes [54].
CONCLUSIONS
Although no established data support EUS-guided transmural drainage of PFC using metal stents over plastic stents, metal stents could be considered at the discretion of the physician, based on operator preference and experience. LAMSs should be considered for walled-off necrosis of the pancreas, because necrosectomy is possible without laborious complicated procedures and thus reduces the risk of morbidities. The development of new stents for various procedures has expanded our ability to effectively drain fluids from the pancreas and gallbladder, approach the bile duct, and perform GJS. Future studies should delineate more clearly which type of therapeutic procedure using metal stents is best suited to a specific disease entity.