INTRODUCTION
Eosinophilic esophagitis (EoE) is defined as an immuneand antigen-mediated chronic esophageal inflammatory disease with eosinophilic accumulation and esophageal dysfunction symptoms, such as vomiting, dysphagia, and food impactions [1]. Although the incidence of EoE is rapidly increasing in Europe and the United States, EoE is a very rare disease in Korea and there have been limited studies on EoE in Korea and other Asian countries [2,3]. The occurrence of EoE is associated with abnormal esophageal epithelial barrier function, and collapse of the esophageal epithelium is one of the most important factors in the development of EoE [1,4]. This abnormality can easily lead to sensitization to food and environmental antigens, followed by cytokine mediated recruitment of eosinophils to the esophagus [1].
Herpes simplex virus (HSV) is a common cause of infectious esophagitis in immunocompromised patients, but HSV esophagitis is also found in immunocompetent healthy children and adults [5-8]. HSV infection in the esophagus can induce the breakdown of the esophageal mucosa and activate an immune reaction that sensitizes the esophagus to foods and aeroallergens [5]. In practice, there have been reports of subsequent occurrences of EoE due to HSV infection [5-7,9-12]. However, active EoE can also cause HSV esophagitis as a result of active untreated EoE disrupting the esophageal mucosa integrity and allowing infectious agents, such as HSV, to invade the esophageal mucosa more easily [10].
The patient described in this report was diagnosed with EoE after esophageal epithelial damage due to HSV esophagitis. Until now, EoE has not been diagnosed after HSV esophagitis in Korea; we report a case where EoE was diagnosed after HSV esophagitis in an immunocompetent pediatric patient, and we present a review of relevant literature on the subject.
CASE REPORT
An 11-year-old boy who had allergic rhinitis without other medical problems was admitted with fever, odynophagia, dysphagia, and chest pain. On examination, his body temperature was 38.2Ōäā and he had multiple ulcers on his lip and hard palate. The initial esophagogastroduodenoscopy (EGD) (Olympus GIF-Q260 Video Gastroscope; Olympus, Tokyo, Japan) revealed numerous depressed linear ulcerative lesions at the mid-esophagus. The longitudinal ulcers had erythematous mucosa with raised margins and yellow bases with a volcano-like appearance at the distal esophagus (Fig. 1A). Polymerase chain reaction (PCR) and immunohistochemistry were performed with the EGD biopsy specimens in order to identify the causative infection of the ulcers.
We extracted genomic DNA from the fresh esophageal biopsy tissues using the QIAsymphony kit (Qiagen, Hombrechtikon, Switzerland), following preparation with tissue lysis buffer and proteinase K solution (Qiagen). We mixed the primer mixture of HSV type 1 and type 2 (BioCore, Seoul, Korea) and the genomic DNA. PCR was performed with a cycling condition consisting of a denaturation step for 12 minutes (95┬░C), followed by 35 cycles of 45 seconds at 94┬░C, 67┬░C, and 72┬░C respectively, and a final extension at 72┬░C for 5 minutes. Electrophoresis was performed with the PCR products and the internal control. The marker of the 279 base pair was HSV type 1 and the 180 base pair was HSV type 2. Although HSV type I was positive in the PCR test, HSV was negative in the immunohistochemistry exam, which also showed nonspecific inflammatory and ulcerative findings without cytopathic changes and intranuclear inclusions (Fig. 1B). We performed PCR with skin, blood, and gastrointestinal tissues of six patients in parallel. HSV type 1 was only positive in the esophageal tissue of this patient, and so we could exclude the possibility of contamination during the test. Cytomegalovirus and Epstein-Barr virus were negative in the PCR test, and there was no evidence of Candida infection in the histopathologic stain. We made a diagnosis of HSV esophagitis, and treated the patient with intravenous acyclovir (5 mg/kg/day) and a proton pump inhibitor (PPI) (1 mg/kg/day). The symptoms improved and we performed an EGD 9 days after admission.
Although the mucosal edema and erythema were improved, the ulcers that were observed in the mid and distal esophagus remained as scars (Fig. 2A). Histopathologic examination showed an improvement in inflammation and ulcerative change, and HSV type 1 was no longer detectable by PCR (Fig. 2B). As a result of these improvements, the patient was discharged 11 days after admission. Investigations to assess immune function, such as measurement of immunoglobulins, complement, and lymphocyte subsets, were normal, and an antibody test for human immunodeficiency virus was negative. The total IgE was increased to 512.7 IU/mL (1.9ŌĆō170.0), and intravenous acyclovir and PPI were administered for 10 days. The intravenous PPI treatment was then changed to an oral medication and used for a total of 8 weeks.
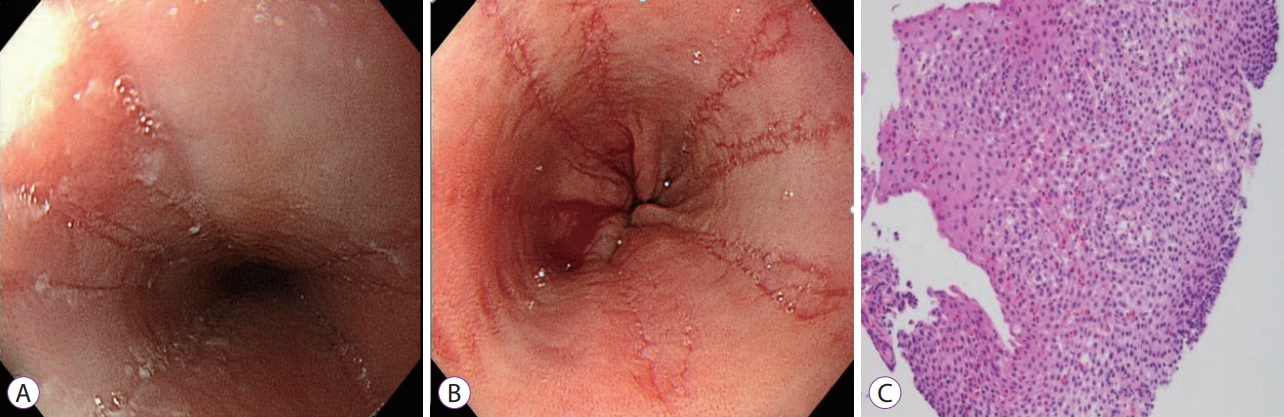
We performed a follow-up EGD at 12 weeks after diagnosis, in which endoscopic findings of EoE with white exudates and multiple linear furrows without mucosal blood vessels in the mid and distal esophagus were identified (Fig. 3A, B). Upon histopathologic examination of the endoscopic biopsies, there was no evidence of HSV infection, and large quantities of intraepithelial eosinophils were observed in the proximal, middle, and distal regions of the esophagus (Fig. 3C). In total, 20, 50, and 200 eosinophils were observed in high-power fields of the proximal, middle, and distal regions of the esophagus, respectively. In the stomach and duodenum, 1 and 15 eosinophils were identified in high-power fields, respectively, and the total blood eosinophil count was increased to 500/mm3. HSV IgG was converted from negative in the initial test, to positive at follow-up, but IgM was negative in both tests.
Although we treated with intravenous acyclovir and PPI for 10 days and oral PPI for the next 7 weeks, the patientŌĆÖs endoscopic and histological findings were compatible with EoE; as such, we made a diagnosis of EoE following HSV esophagitis. Although the patientŌĆÖs chest pain and odynophagia resolved after treatment, he still experienced epigastric pain with meals. We intend on starting a six food elimination diet followed by a topical steroid if a food elimination diet confirms intolerance.
DISCUSSION
Currently, the incidence of EoE is approximately 10 cases per 100,000 persons worldwide, and Caucasians are more affected by EoE than any other race [3,13]. Asian countries have not undergone population-based studies of EoE because of the rareness of the disease in Asians. Indeed, Kim et al. [14] reported that in a single center study in Korea the percentage was 0.23% among patients who received EGD over an 8 year period. There have been no studies on pediatric EoE in Korea. The presented case is significant because of the rarity of EoE following HSV esophagitis in Korea.
EoE is characterized by esophageal dysfunction and esophageal eosinophilia due to complex mechanisms, such as genetic and environment factors, and antigenic stimuli from food or aeroallergens [4]. This immune response is mediated by T helper 2 (Th2) lymphocytes, which secrete various cytokines that are responsible for eosinophil recruitment into the esophagus, and subsequent disruption of the epithelial barrier [4]. The impaired esophageal barrier is one of the known factors associated with the occurrence of EoE.
HSV esophagitis is common in immunocompromised patients, but has also been continuously reported in immunocompetent adult and pediatric patients [8]. As in this case, patients with HSV esophagitis present with retrosternal pain and odynophagia with or without fever [11]. Endoscopic findings of HSV esophagitis demonstrate circumscribed ulcers with raised edges and erythema, described as volcano-like ulcers [11]. In the histopathological diagnosis of HSV esophagitis, acute inflammation, ulceration, and cytopathic changes are major points [15]. A definitive histological diagnosis of HSV esophagitis requires herpes virus isolation from the tissue during cell culture [15]. Although viral culture offers high sensitivity and specificity, transport conditions and laboratory environmental factors have an effect on virus isolation [8]. In addition, biopsies should be taken from the ulcer margins in order to increase the HSV isolation rate [8]. On the other hand, the PCR test has a higher detection rate for HSV than cell culture [8]. Indeed, in the described case, HSV was only detected by PCR and not from immunohistochemical examination. We believe that the histological features of HSV infection were not clearly observed in this case because the site of the biopsies was the base of the ulcer.
The link between EoE and HSV esophagitis has been reported in some cases in Western countries [5-7,10-12,16-18]. There has been debate as to whether HSV esophagitis is the causative agent of EoE, or whether EoE is a predisposing factor for HSV esophagitis. Esophageal tissue injury due to HSV infection may cause a breakdown of the esophageal mucosal barrier, resulting in the development of hyper-reactivity to food and environmental antigens [11,12]. This change may also trigger Th2 cell clones, which induce cytokine secretion and eosinophilic inflammation [11]. Additionally, EoE might already be present prior to HSV infection, because the esophageal eosinophilic mucosal inflammation in EoE is susceptible to candidiasis, HSV, and other viral infections [11]. In this case, HSV infection occurred first, followed by EoE. We found 18 cases related to EoE and HSV esophagitis reported in PubMed Central (Table 1); in these cases, the mean age of EoE occurrence was 16.7┬▒7.3 years (4ŌĆō29 years), and HSV infection preceded EoE in 12 of 18 cases. HSV esophagitis and EoE were simultaneously detected in three cases, and in the remaining three cases, HSV esophagitis was diagnosed following EoE. However, it is difficult to clearly identify the relationship between EoE and HSV esophagitis. Acute inflammation as a result of HSV infection can induce pathologic findings that are dominated by neutrophils, and EoE may be masked if there is an underlying disease. HSV esophagitis is more likely to occur with inflammation of the esophageal mucosa, as in EoE, because HSV esophagitis is rare in individuals with normal immunity. However, as in this case, there have been reported cases of EoE following HSV infection. In the current case, the child had no previous EoE symptoms, but EoE occurred following HSV esophagitis, with ulceration in the mouth and gingiva. Through several cases that have been previously reported, we found that the mean duration of EoE occurrence following HSV esophagitis was 8 weeks. In the current case, EoE was detected by EGD 12 weeks after the diagnosis of HSV esophagitis.
Thus, we report a case of HSV esophagitis in a healthy child with normal immune function. It is not yet established whether HSV esophagitis is a causative factor for EoE, or whether EoE is a predisposing factor for HSV esophagitis. However, if HSV esophagitis is confirmed, endoscopic follow-up at 8 weeks should be considered in order to establish whether EoE is present.