INTRODUCTION
RATIONALE BEHIND AQEU ASSESSMENT ITEMS FOR ENDOSCOPIC SEDATION


AbstractSedation, defined as the depressed level of consciousness, induced by drug administration, is widely used for gastrointestinal endoscopy to relieve a patientŌĆÖs anxiety and discomfort. In addition, successful procedure is anticipated with control of unintended movements. Endoscopic sedation, however, cannot be free from the risk of serious adverse events, e.g., cardiopulmonary compromise. Therefore, principles on personnel, facility and equipment, as well as performance itself, should be followed to prevent unfavorable incidents. In this article, sedation guidelines for the Accreditation of Qualified Endoscopy Units, issued by the Korean Society of Gastrointestinal Endoscopy, are presented.
INTRODUCTIONSedation is defined as the depressed level of consciousness induced by administration of drugs. When gastrointestinal endoscopy is performed, sedation is frequently used for the sake of both the patient and the endoscopist. For a patient, anxiety and discomfort can be relieved. Moreover, amnesia, regarding memory of the event, may be anticipated. For an endoscopist, the quality of the examination is improved through the control of unintended movements. The level of sedation is a continuum ranging from minimal sedation (anxiolysis) to general anesthesia, which is defined by a patientŌĆÖs degree of spontaneous ventilation, maintenance of cardiovascular function, and response to verbal and tactile stimuli (Table 1).1 The targeted level of sedation may differ depending on a patientŌĆÖs condition and the duration or complexity of the procedure. Usually, moderate sedation is the preferred level. Deep sedation, however, may be achieved with or without intention, in which case the risk of serious adverse events (AEs), e.g., cardiopulmonary compromise, increases. In this article, sedation guidelines for the Accreditation of Qualified Endoscopy Units (AQEUs), issued by the Korean Society of Gastrointestinal Endoscopy (KSGE), are presented (Table 2).
RATIONALE BEHIND AQEU ASSESSMENT ITEMS FOR ENDOSCOPIC SEDATION Pre-procedure assessment: 4 items
1. Confirmation of medical history and American Society of Anesthesiologists physical status classification
Prior to endoscopic sedation, the medical history of the patient must be verified and the physical status of the patient must be assessed according to the American Society of Anesthesiologists (ASA) physical status classification system.
Rationale: Prior to endoscopic sedation, previous medical history of the patient, including cardiopulmonary disease, neurological disease, sleep apnea, AEs from using sedatives, current medication, alcohol abuse, and compliance with fasting time, must be checked to determine if any of these factors may affect the outcome of sedation [1,2]. The findings must be recorded in the pre-screening chart or pre-procedure assessment report (Supplementary file 1).
2. Vital signs
Prior to endoscopic sedation, the patientŌĆÖs blood pressure, heart rate, respiratory rate, and oxygen saturation must be recorded.
Rationale: Sedatives and analgesics administered during endoscopic sedation may cause cardiovascular and/or respiratory AEs, such as a temporary drop in blood pressure, respiratory depression, or arrhythmia. In particular, greater caution should be taken in cases comorbid with chronic diseases. Accordingly, pre-procedure assessment on these factors is necessary, and appropriate measures should be taken when required.
3. Informed consent and guardian
A recommended format of the consent form must be used, or all information needed for sedation consent must be included. The patient must be notified regarding the possible need of being accompanied by a guardian during endoscopic sedation and the actual presence of this individual must be confirmed.
Rationale: The endoscopic sedation consent form must include an explanation on the sedative endoscopic procedure; purpose and necessity of sedation; processes and methods of endoscopic sedation; problems, AEs, and sequelae associated with endoscopic sedation; alternative treatment options besides endoscopic sedation; and prognosis, if left untreated (Supplementary file 2). Even after an endoscopic sedation has been completed, the patient may still face the risk of AEs associated with the sedative(s) used. Although the patient is discharged after the state of recovery is adequately assessed, each individual may experience a different duration of sustained sedative effect. Therefore, it is necessary for the patient to be accompanied by a guardian when being discharged.
4. Sedation-related education
Rationale: Life-threatening AEs, such as cardiopulmonary dysfunction, may occur during endoscopic sedation; thus, endoscopists and other assistants must have completed sedation-related education on symptoms to check prior to the procedure, monitor during the procedure, and after the procedure, and on emergency measures to ensure patient safety.
Intra-procedure assessment: 3 items
1. Vital signs monitoring
During endoscopic sedation, monitoring and recording the patientŌĆÖs blood pressure, heart rate, respiratory rate, and oxygen saturation at regular intervals is recommended.
Rationale: During the procedure, the patientŌĆÖs blood pressure, heart rate, respiratory rate, and oxygen saturation must be monitored regularly, to discern possible endoscopic sedation-related AEs [3]. The findings must be recorded in the sedation report (Supplementary file 3).
2. Drug administration records
Rationale: An endoscopy findings report serves as evidence of the most important outcomes in endoscopy. Therefore, records of sedatives, analgesics, spasmolytics, and antagonists used during the endoscopic procedure must be available. The use of drugs that are managed under narcotics control is to be confirmed from the narcotics control records, and the use of non-narcotics could be found in relevant records. Records contained in computer storage media or hand written reports are acceptable.
3. Drug administration guidelines
Rationale: The patientŌĆÖs age, health status, current medication, tension, and sensitivity to pain, are factors that may affect the efficacy of sedation, and thus, must be considered prior to endoscopic sedation. Because the required dose of sedatives used for endoscopic sedation may change depending on the patientŌĆÖs general condition, age, and current medication, it is necessary to have sedative administration guidelines in place. In this context, the guidebook for endoscopic sedation issued by the KSGE recommends the following administration method:
When administering midazolam alone to healthy adults aged <65 years, 2.5ŌĆō3 mg of midazolam (or 0.05 mg/kg of body weight) should be slowly administered intravenously, followed by repeated administration of 1ŌĆō2 mg (or 0.02ŌĆō0.03 mg/kg) in 2- to 3-minute intervals until adequate sedation is achieved. For general diagnostic examinations, a dose within 5 mg should be sufficient and a higher dose is not recommended since it increases the risk of AEs. For patients with a high risk of endoscopic sedation-related AEs, such as the elderly aged Ōēź65 years, patients with ASA class III or higher, and patients with airway obstruction, a dose reduction of Ōēź20% should be applied. Co-administering midazolam with narcotic analgesics has a synergistic effect, and thus, the injection should be accompanied by a single administration of midazolam reduced by 20% from the dose described above. When using meperidine, adequate sedation could be achieved with an initial intravenous injection of 12.5ŌĆō25 mg, followed by repeated administration of 12.5ŌĆō25 mg in 3- to 5-minute intervals when necessary. If the length of the procedure is short, a single injection is sufficient and the maximum dose of 50 mg should not be exceeded. When using fentanyl, an initial intravenous injection of 25ŌĆō50 ╬╝g should be used, followed by repeated administration of 12.5ŌĆō25 ╬╝g in 3- to 5-minute intervals when necessary. However, a single injection is sufficient in most cases and the maximum dose of 100 ╬╝g should not be exceeded. For patients with high risk of endoscopic sedation-related AEs, elderly patients aged Ōēź65 years, or patients with ASA class III or higher, the analgesic dose should be reduced by Ōēź50% (meperidine 12.5 mg/fentanyl 25 ╬╝g).
When administering propofol alone to healthy adults aged <65 years, 30ŌĆō40 mg (or 0.5 mg/kg) should be administered, and an additional 10ŌĆō20 mg should be administered with at least a 20-second intermission, as necessary. For patients with a high risk of endoscopic sedation-related AEs, elderly aged Ōēź65 years, or patients with ASA class III or higher, the administered dose should be reduced by Ōēź50%. When opting for a balanced propofol sedation, midazolam (0.025ŌĆō0.05 mg/kg), meperidine 25 mg (or fentanyl 50 ╬╝g), and propofol (10ŌĆō20 mg or 0.25 mg/kg) should be administered in sequence to healthy adults aged <65 years; sedation should be maintained by repeated administration of 10ŌĆō20 mg of propofol with at least 20-second intermission. For patients with high risk of endoscopic sedation-related AEs, elderly aged Ōēź65 years, or patients with ASA class III or higher, the administered dose should be reduced by Ōēź50% or midazolam should be used alone.
Post-procedure assessment: 3 items
1. Dedicated nurses
Rationale: Nurses assigned to the recovery room are needed for proper patient monitoring following endoscopic sedation, assessment of discharge criteria, and for providing adequate explanation to the patient and guardian at the time of discharge.
2. Patient monitoring
After endoscopic sedation, the patientŌĆÖs oxygen saturation and heart rate must be monitored and blood pressure must be measured regularly from the time of admission to the recovery room until the time of discharge.
Rationale: There are individual differences with respect to recovery from endoscopic sedation, and in rare cases, AEs, such as respiratory distress, apnea, hypotension, bradycardia, and shock, may occur. Therefore, the patientŌĆÖs oxygen saturation, heart rate, and blood pressure must be monitored and even electrocardiogram (ECG) should be performed when necessary, until discharge in accordance with the guidelines. Therefore, patient monitoring and oxygen-supply equipment must be available and countermeasures need to be established.
3. Discharge criteria
After endoscopic sedation, the patient must be discharged in accordance with the designated criteria.
Rationale: After endoscopic sedation, the risk of AEs associated with the sedative used still remains. Therefore, standards and manuals for patient care in the recovery room and discharge from the recovery room are absolutely necessary. The most commonly used recovery assessment scales include the Aldrete score (Table 3) and the Postanesthetic Discharge Scoring System (PADSS) [4].
Narcotics control: 2 items
1. Drug management
Narcotics used for endoscopic sedation must not be easily accessible by patients and must be stored in a narcotics storage facility with a lock.
Rationale: Narcotics act on the human central nervous system and are recognized to cause serious harm to the human body when misused or abused. Therefore, they must be kept in a reserved area that is not easily accessible by patients and in an area protected by a double lock to avoid misuse and abuse.
2. Medication preparation
Narcotics must be dispensed immediately before administration and prepared in an independent area, such as a medication preparation room.
Rationale: Close attention must be paid to the storage of narcotics since they have a high risk of theft and loss, which may lead to misuse and abuse. Drugs containing a lipid emulsion must be dispensed and prepared immediately before the administration owing to a potential risk of infection due to bacterial contamination.
 Pre-procedure assessment: 3 items
1. The ratio of examination room beds to recovery room beds
Rationale: Emergencies may occur during endoscopic sedation. Accordingly, areas, equipment, and personnel for monitoring and responding to such situations are needed [5].
2. Verification of pre-procedure Mallampati score
Before beginning the endoscopic sedation, it is recommended to predict the risk of hypoxia and difficulty of endotracheal intubation based on the patientŌĆÖs Mallampati score.
Rationale: To determine cases with respiratory depression severe enough to require endotracheal intubation, the Mallampati score should be assessed prior to endoscopic sedation to determine the risk of hypoxia and the expected degree of difficulty when intubating the patient. The Mallampati classification is as shown below (Fig. 1) [6]. For cases characterized as Class IV, assistance from an anesthesiologist may be required [3].
3. Assessment of level of consciousness
Rationale: Endoscopic sedation involves administration of sedative(s) that can lower the patientŌĆÖs level of consciousness for the procedure. Therefore, it is necessary to assess the patientŌĆÖs level of consciousness accurately, prior to the procedure and determine whether sedation could be performed. An example of assessment of the level of consciousness is as follows (Table 4) [7]:
Intra-procedure assessment: 1 item
1. ECG monitoring
ECG monitoring during endoscopic sedation is recommended in patients with serious cardiovascular disease or arrhythmia.
Rationale: When performing moderate or deep sedation on patients with serious cardiovascular disease or arrhythmia, continuous ECG monitoring is recommended. It may also be helpful in cases involving elderly patients or patients with respiratory disease, and when the procedure is expected to prolong.
Post-procedure assessment: 1 item
1. ECG monitoring
ECG monitoring after endoscopic sedation is recommended in patients with serious cardiovascular disease or for those who showed unstable vital signs during the procedure.
Rationale: ECG monitoring should be performed on patients with serious cardiovascular disease or arrhythmia or in cases for which it is recommended. It may also be helpful in cases involving elderly patients or patients with respiratory disease, when bradycardia or arrhythmia occurs during the procedure, and when the patient shows unstable vital signs during the procedure.
Fig.┬Ā1.The Mallampati classification. Grade I: soft palate, fauces, uvula, pillars are visible. Grade II: soft palate, fauces, portion of uvula are visible. Grade
III: soft palate, base of uvula are visible. Grade IV: only hard palate is visible
(Modified from Mallampati et al. [6]). 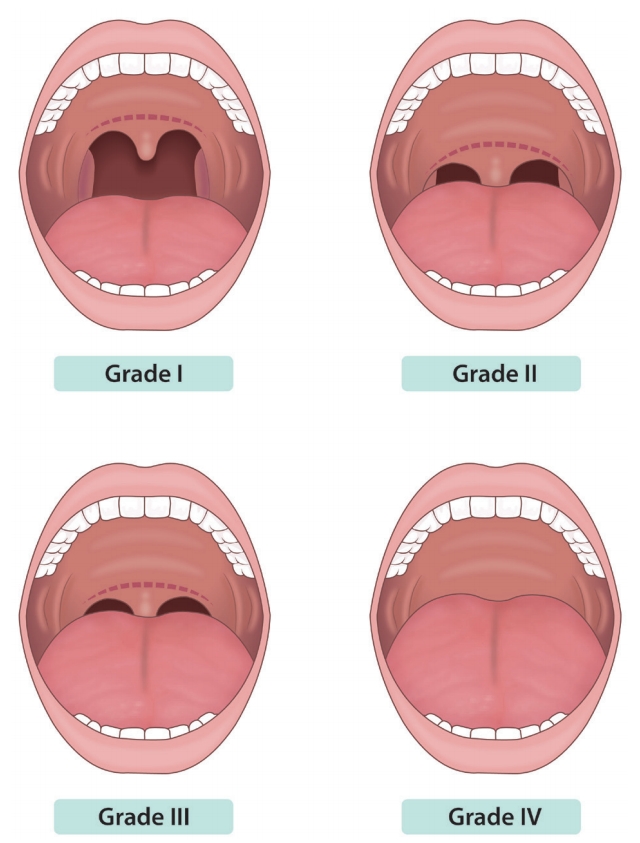
Table┬Ā1.Levels of Sedation and Anesthesia Modified from American Society of Anesthesiologists Task Force on Sedation and Analgesia by Non-Anesthesiologists. [1] Table┬Ā2.Accreditation of Qualified Endoscopy Unit Assessment Items for Endoscopic Sedation Table┬Ā3.Adult Post Procedure/Post Sedation Recovery (Aldrete) Score Table┬Ā4.Modified ObserverŌĆÖs Assessment of Alertness and Sedation (MOAA/S) Scale Modified from Chernik et al. [7] REFERENCES1. American Society of Anesthesiologists Task Force on Sedation and Analgesia by Non-Anesthesiologists. Practice guidelines for sedation and analgesia by non-anesthesiologists. Anesthesiology 2002;96:1004ŌĆō1017.
2. Cohen LB, Delegge MH, Aisenberg J, et al. AGA Institute review of endoscopic sedation. Gastroenterology 2007;133:675ŌĆō701.
3. ASGE Standards of Practice Committee, Early DS, Lightdale JR, et al. Guidelines for sedation and anesthesia in GI endoscopy. Gastrointest Endosc 2018;87:327ŌĆō337.
5. Mulder CJ, Jacobs MA, Leicester RJ, et al. Guidelines for designing a digestive disease endoscopy unit: report of the World Endoscopy Organization. Dig Endosc 2013;25:365ŌĆō375.
|
|
|||||||||||||||||||||||||||||||||||||||||||
