INTRODUCTION
Colorectal cancer is rare in teenagers, especially without known risk factors such as inflammatory bowel disease, familial adenomatosis polyposis, and hereditary nonpolyposis colon cancer (HNPCC). Although the presenting features of colorectal carcinoma are similar between adolescents and adults, it is frequently overlooked in the differential diagnosis in young patients.1 Many reports suggest that children are more likely than adults to have advanced-stage disease at presentation, unfavorable tumor histology, and poor outcome.2
In the pathological point of view, a mucinous histology is considerably more frequent in children and adolescents than in adults. Here, we report a case of mucinous adenocarcinoma of the colon in a 19-year-old male patient without any risk factors. We also review the clinical and pathological characteristics of young-age sporadic colorectal cancer (YSCC).
CASE REPORT
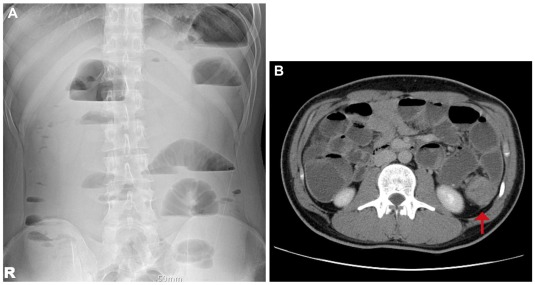
A 19-year-old man visited the gastroenterology outpatient department complaining severe left abdominal pain that developed 1 month ago. On physical examination, he was found to have a distended abdomen with severe tenderness on the left lower quadrant. An abdominal X-ray revealed a multiple stepladder sign (Fig. 1A). Abdominal computed tomography scan was then immediately performed which revealed a distal descending colon mass causing mechanical obstruction (Fig. 1B).
The patient had been living in Australia for his studies. He first felt a left-sided abdominal pain a month ago. He also had acute constipation and passed loose stool 1 to 2 times per week. He visited a private clinic in Australia, and the physician gave a diagnosis of acute colitis with regard to his abdominal pain and loose stool. Despite medication, he did not feel any symptomatic improvement so he returned to South Korea the previous day.
The patient was sent to an endoscopy room, and an emergency colonoscopy showed a large, fungating mass obstructing lumen at 40 cm from the anal verge. Through-the-scope stent insertion was performed to decompress the gastrointestinal tract (Fig. 2). After stenting, a massive stool gushed out. No other polyp or mass was seen on the sigmoid colon and rectum.
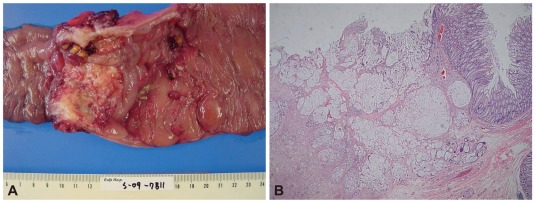
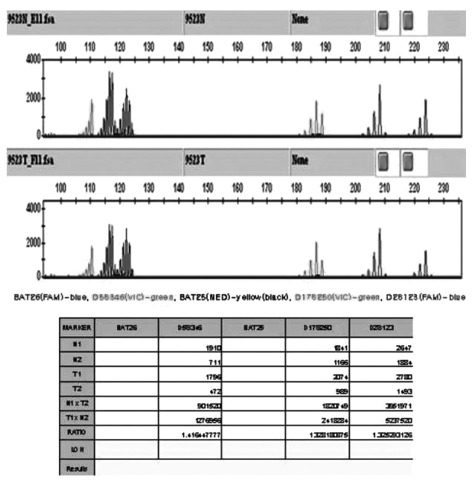
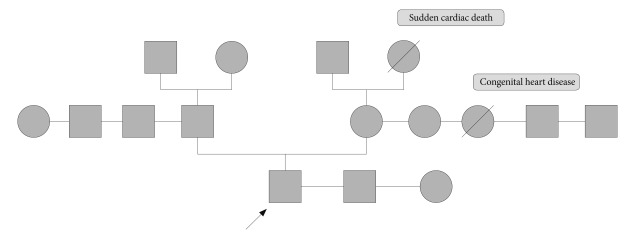
On the second hospital day, his abdominal pain markedly improved. His serum carcinoembryonic antigen level was 3.85 ng/mL, and his erythrocyte sedimentation rate and C-reactive protein level were elevated (13 mm/h and 3.31 mg/dL, respectively). No other abnormal laboratory findings, such as anemia, or leukocytosis, were observed. Biopsy of the colonic mass suggested a mucinous adenocarcinoma. Positron emission tomography scan revealed a hypermetabolic lesion on the descending colon without other metastatic lesions. The patient was transferred to the general surgery department and was subjected to left hemicolectomy under general anesthesia. The pathology was confirmed as mucinous adenocarcinoma (7.0├Ś4.5 cm), penetrating the visceral peritoneum and with one regional lymph node involvement out of 47 (Fig. 3). For HNPCC evaluation, immunohistochemical staining for MLH1 and MSH2 was done, which gave a normal result. RT-PCR analysis did not detect microinstability in any of the markers tested (Fig. 4). The patient had no family history of cancer, including his parents, grandparents, and cousins (Fig. 5). He was discharged after surgery without any events. He was transferred to other hospital for adjuvant chemotherapy according to his family's decision.
DISCUSSION
Colorectal carcinoma rarely occurs in children and adolescents. It is generally considered to be a disease of older persons; more than 90% of colorectal cancer patients is above 55 years old.3 Groups of young patients known to be at increased risk for colorectal carcinoma are those with inflammatory bowel disease, HNPCC, and polyposis syndromes of the gastrointestinal tract.4
APC gene mutations produce classic or attenuated familial polyposis coli.
Mutations in mismatch repair (MMR) genes, principally MSH2 and MLH1 but also PMS1 and PMS2, cause HNPCC.5 The Amsterdam criteria, the most widely used diagnostic criteria for HNPCC, have the following requirements: 1) there should be 3 or more family members with histologically verified colorectal cancer, 2) with 1 member being a first-degree relative of the other two, 3) at least 1 case should have been diagnosed before the age of 50 years, and 4) 2 or more generations should be involved.6
Most patients with clinically diagnosed HNPCC will have germline MMR gene mutations. Although identification of patients with germline MMR gene mutations in the absence of a family history is important, most young colorectal cancer patients without a family history of HNPCC are unlikely to have germline MMR gene mutations.5
Both HNPCC and YSCC are considered to arise through the mutator phenotype genetic pathway of colorectal carcinogenesis, although YSCC may also be caused by hypermethylation of the MMR gene MLH1 instead of gene mutation itself.4
Several observations have suggested that chromosome 14 could play an important role in microsatellite stable (MSS) colon carcinogenesis. As loss of chromosome 14 is more frequent in aggressive tumors and in young patients, this gene location is likely responsible for tumor aggressiveness and hereditary predisposition.7
We reported this case as an YSCC because the patient had neither family history of colon cancer nor any other malignancy, and showed MSS without any germline abnormality. He had no risk factors such as inflammatory bowel disease or multiple colon polyps.
YSCC also presents features similar to adult colorectal cancer, such as abdominal pain, altered bowel habits, weight loss, and rectal bleeding.1 However, young patients present with more advanced-stage disease at diagnosis compared with older patients. This may be because of delayed diagnosis and poorer pathological findings.3 In young patients who develop colorectal carcinoma but have no known predisposing genetic risk factors, late diagnosis may result from the clinician's failure to consider the possibility of malignant disease in the differential diagnosis.4
Another distinct feature of YSCC is its pathological characteristics. It has a higher proportion of patients with multiple nodal metastasis, distant metastasis, and poorly differentiated tumors. YSCC also shows aggressive tumor biology, including a mucinous component, signet-ring cell carcinoma, and perineural, vascular, and lymphatic invasion.
Mucinous adenocarcinoma is a distinct subgroup of adenocarcinoma and comprises about 10% to 15% of all colorectal carcinomas.8 However, an average of 28% of the lesions found in younger patients constituted mucinous tumors compared with an average of 5% for adults with colorectal carcinoma.9
Mucinous adenocarcinoma shows higher frequencies of poor differentiation, advanced tumor stage, loss of MMR expression, and increased MUC2 expression compared with non-mucinous adenocarcinoma. These discrepancies might vary across different subgroups of colorectal cancer patients. According to a recent report, HNPCC patients showed a similar incidence of loss of MMR expression, and YSCC patients showed a similar proportion of advanced tumor stage between mucinous and non-mucinous adenocarcinoma. In addition, the tumor locations of mucinous adenocarcinoma are significantly different between HNPCC (predominantly in the right colon) and YSCC (predominantly in the left colon and rectum).8
The diagnostic and treatment tools for YSCC are same as those used for adult colon cancer. However, a report suggested that exploratory laparotomy yields a higher proportion of diagnostic result for colorectal carcinoma in young patients than in adults. Early colonoscopy is less frequently performed in young patients than in older patients.1 Our case was also initially misdiagnosed as simple enteritis without any study during the first one month even though presenting significant symptoms.
In a review of young-age colon cancer, studies comparing stage-for-stage survival found that young patients with Dukes' stage A or B tumors appear to have better survival than older patients with similar-stage disease. Young patients with Dukes' C or D lesions do the same or worse than older patients with the same disease stages.3 A large cohort study found that young patients showed poor outcome despite the similarity of their treatment to the standard therapy for adult colorectal carcinoma,10 reflecting the preponderance of advanced-stage disease in young patients. Other factors for the outcome besides advanced-stage were incomplete resection, mucinous histology and proportion of signet ring cells.1
A prospective national cohort study following standardized total mesorectal excision for rectal cancer in patients aged less than 40 also showed considerably lower survival rate. The authors suggested that biologically more aggressive cancer rather than diagnostic delay seemed to account for the advanced stage at the time of diagnosis, leading to poor survival rates. Thus, age may be a significant and independent prognostic factor determining metastasis and survival in rectal cancer. Patients younger than 40 years of age had 5 times higher risk of metastasis and 6 times higher risk of death compared with patients between 40 and 44 years of age. The youngest rectal cancer patients seem to have additional negative prognostic factors, not related to histopathologic findings or tumor stage.11
In conclusion, we reported a case of a 19-year-old male patient with colon obstruction due to sporadic colon cancer with a mucinous histology. He had absolutely no predisposing conditions. His cancer diagnosis was delayed by the physician's failure to consider malignancy in the differential diagnosis. Therefore, even in low-risk young patients, symptoms such as unexplained abdominal pain, rectal bleeding, or changes in bowel habits should be considered representative of significant colorectal lesions. This case warrants increased awareness and aggressive pursuit of symptoms in young patients without any risk factors.