INTRODUCTION
Schwannoma of the gastrointestinal (GI) tract is considered to be a rare and distinctively different neoplasm from conventional schwannomas that arise in soft tissue or the central nervous system. Histologically, GI schwannomas are S-100 protein-positive spindle cell tumors with a microtrabecular pattern, peripheral lymphoid cuffing, and occasional germinal centers.1 They are benign tumors associated with an excellent prognosis after surgical resection as reported by Daimaru et al.2 GI schwannomas occur most commonly in the stomach (60% to 70% of cases), followed by the colon and rectum.1 Esophageal and small intestinal schwannomas have been rarely reported.2
Endoscopic ultrasound (EUS)-guided biopsies are reliable, safe, and effective techniques in obtaining samples for cytological or histological examinations either as a primary procedure or in cases that other biopsy techniques have failed. EUS-guided fine needle aspiration biopsy (EUS-FNA), as well as EUS-guided Trucut biopsy (EUS-TCB), has been proven to be of significant value in the diagnostic evaluation of benign and malignant diseases, as well as in staging of the malignant tumors of the GI tract and of adjacent organs.3 This is a very rare case report of gastric schwannoma diagnosed by EUS-TCB to our knowledge.
CASE REPORT
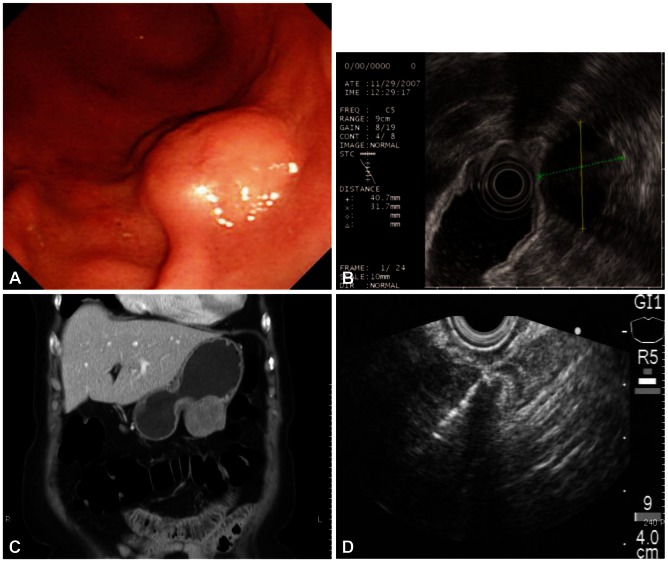
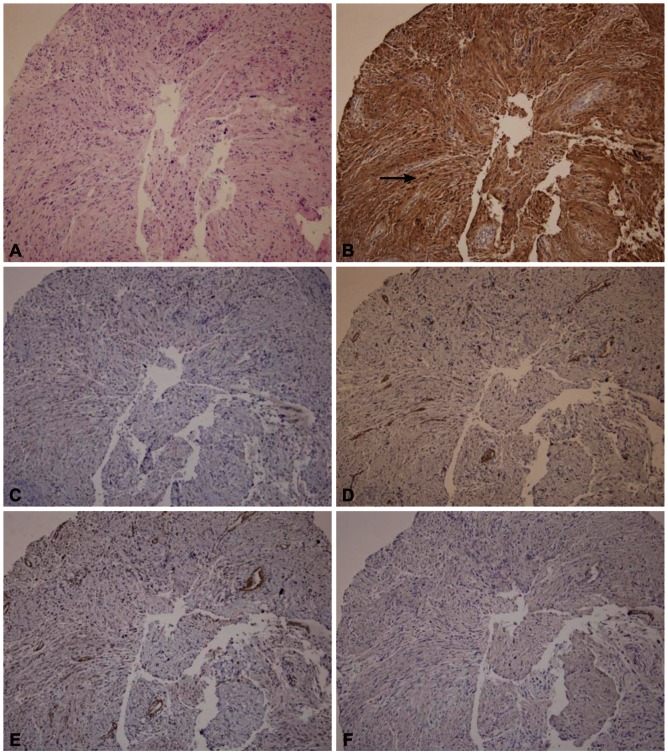
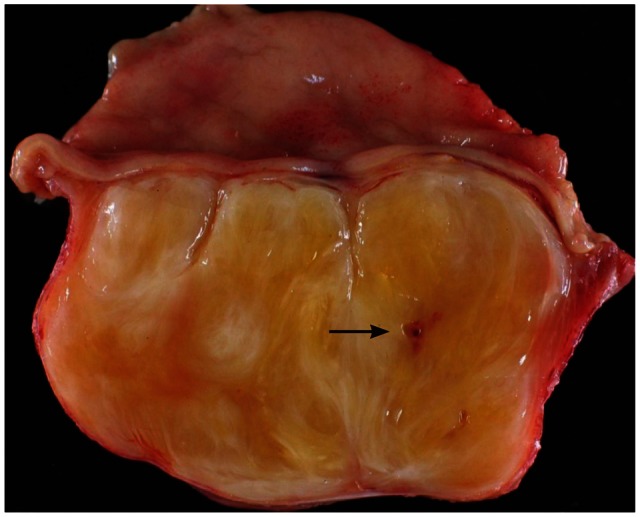
A 72-year-old female was referred for the evaluation of right colon cancer. During staging workup, esophagogastroduodenoscopy showed submucosal elevated lesion with bridging folds located at the greater curvature side of the antrum (Fig. 1A). EUS (UM-2000; Olympus, Tokyo, Japan) demonstrated a 40.7У31.7 mm-sized round, hypoechoic mass with internal hyperechogenicity originating from the proper muscle layer (Fig. 1B). Abdominal computed tomography scan showed 5У4 cm-sized heterogenous mass located at the greater curvature of the stomach without enlarged lymph nodes (Fig. 1C). EUS-TCB with a 19 gauze needle (QuickCore; Wilson-Cook Medical Inc., Winstom-Salem, NC, USA) was performed (Fig. 1D). The tissue from the tumor was composed of spindle cells that were only stained with S-100 on immunohistochemistry. Staining for CD 117, CD34, smooth muscle actin, and desmin was negative. These results corresponded with schwannoma of the stomach (Fig. 2). Laparoscopic gastric wedge resection was performed with right hemicolectomy of the colon cancer. There was an intramural solid mass from muscularis propria of the stomach, measuring 5.5У3.5 cm. On section, the cut surface showed a yellowish gray and myxoid appearance (Fig. 3). On immunohistochemistry, diffuse and intense positivity for S-100 protein indicating neurogenic differentiation of the tumor cells was observed. The tumor did not show any expression of CD117, CD34, smooth muscle actin, and desmin, identical with the result of TCB. This suggested that final pathologic diagnosis was also gastric schwannoma.
DISCUSSION
Schwannomas arise from Schwann cells of the nerve sheath, which encompass the axons of peripheral nerves. Schwannomas of the GI tract have rarely been reported and occurred predominantly in the stomach. Their reported prevalence ranges from 3.3% to 12.8% of all GI mesenchymal tumor.1,2 In contrast to gastric GI stromal tumors (GISTs), which may be malignant or have malignant potential, schwannomas behave in a benign fashion and there have been no recurrence, metastasis, or tumor-related mortality reported after curative resection.1
EUS is a useful technique to further characterize subepithelial lesions and surrounding structures of the GI tract.4,5 It can be verified to ascertain the layer of tumor origin and differentiate intramural lesions from extrinsic compressions. EUS measurements of tumor size, cystic spaces, and extraluminal margins have a positive predictive value in differentiating between benign and malignant submucosal tumors.6 Ji et al.7 reported the positive predictive value of 98.7% for locating GI mesenchymal tumors with EUS and 80.3% positive rate of differentiation between benign and malignant tumors using EUS.
It is also difficult to obtain tissue specimens from muscularis propria or muscularis mucosa by conventional endoscopic biopsy. These types of tumors, which cannot be clearly diagnosed with EUS, require EUS-FNA or EUS-TCB for better evaluation. Preoperative diagnosis using EUS-FNA or EUS-TCB may be improved with the application of immunohistochemical analysis. As was in our case, subepithelial tumors originating from the proper muscle layer of the stomach are GIST, leiomyoma, etc. All these subepithelial tumors showed hypoehic lesions originating from the proper muscle layer in the EUS. EUS imaging alone cannot make a differential diagnosis among these subepithelial tumors. Thus, obtaining tissues from submucosal tumor with EUS-FNA or TCB and performing immunohistochemical analysis will help to make accurate diagnoses and improve patient prognosis.8,9 In a recent study, FernУЁndez-Esparrach et al.10 evaluated the diagnostic yield of EUS-FNA and EUS-TCB in 40 patients with gastric subepithelial lesions. The diagnostic yield of EUS-FNA was 52% and that of EUS-TCB was 55%, because of technical failure and inadequate sample. They are reported cases that were diagnosed by EUS-FNA as mediastinal schwannoma11 and retroperitoneal schwannoma.12 Until now, gastric schwannoma diagnosed by EUS-FNB are reported seldom.
Spindle cell neoplasms of the GI tract show considerable morphologic overlap. Thus, immunohistochemistry is an essential tool for proper diagnosis of these lesions. Several immunohistochemical stains are particularly helpful in the evaluation of these spindle cell lesions, each with its own strengths and limitations. The strength of S-100 protein expression in spindle cell neoplasms of the GI tract lies in its ability to identify schwannomas. Schwannomas show strong and uniform nuclear and cytoplasmic staining for S-100 protein in 100% of cases in reported series.13-16 In this case, the tumor was successfully diagnosed as gastric schwannoma by EUS-TCB with immunohistochemistry and curative resection was performed by laparoscopic surgery.