INTRODUCTION
Gastric subepithelial tumors (SETs) are commonly encountered during upper endoscopy. Gastric SETs can comprise different types of abnormal growth underneath the mucosa of the stomach and can include the following: gastrointestinal stromal tumor (GIST); leiomyoma; schwannoma; granular cell tumor; inflammatory fibroid polyps; lipoma; and ectopic pancreas. Approximately 1 in every 300 routine endoscopies results in a diagnosis of SETs of the gastrointestinal wall [1]. Sixteen percent of gastric SETs with pathologic verification were diagnosed with malignant disease [2]. Imaging studies such as endoscopic ultrasound (EUS) have been found to be limited in distinguishing between malignant and nonmalignant gastric SETs. Therefore, pathological diagnosis of gastric SETs is necessary.
Several techniques for pathological diagnosis have been proposed. These include stacked biopsy, EUS-guided fine-needle aspiration (EUS-FNA), and EUS-guided trucut biopsy (EUS-TCB). Unfortunately, the diagnostic yield of the stacked biopsy is only approximately 17% to 38% [3,4]. EUS-FNA is considered to be the most useful method for obtaining a tissue diagnosis [5]. The diagnostic yield of EUS-FNA varies from 43.3% to 83.9% [6-8]. In addition, it has been proven to be limited in obtaining tissue samples sufficient for immunohistochemical analysis, which is essential for the diagnosis of SETs of mesenchymal origin. Although EUS-TCB had been expected to provide an increase in diagnostic yield, a recent study by Fernandez-Esparrach comparing EUS-FNA and EUS-TCB found that both procedures had similar diagnostic yield [9]. Moreover, due to the high cost of endosonographic equipment, EUS-FNA and EUS-TCB are only available at a few medical institutions.
In this paper, we describe a method for pathological diagnosis of gastric SETs that, involves mucosal incision and forceps biopsy (MIFB).
MATERIALS AND METHODS
Between November 2011 and September 2014, 12 consecutive patients who underwent MIFB for pathological diagnosis of gastric SETs at Gangneung Asan Hospital were enrolled into the study. The procedure and pathology reports of the study participants were reviewed and demographic characteristics were retrospectively abstracted from medical records. The study was approved by the Institutional Review Board of Gangneung Asan Hospital (IRB No. 2014-029).
The patient was sedated using midazolam and meperidine. An initial bolus of 2 to 4 mg and 25 mg meperidine was administered. After injection, the sedation level was evaluated and another 1 to 2 mg was administered if the level was unsatisfactory. Additional sedation and analgesia were titrated after careful consideration by the physician.
Gastric SETs were evaluated by using a radial scanning EUS (GF-UM260; Olympus Medical Systems Corp., Tokyo, Japan). All procedures were performed using an endoscope equipped with a water-jet system and a transparent hood (Q260; Olympus Medical Systems Corp.). A hook knife (Olympus) and an electrosurgical unit (VIO 300D; ERBE, T├╝bingen, Germany) were used for mucosal incision. A coagrasper (Olympus) was used for hemostasis during the procedure.
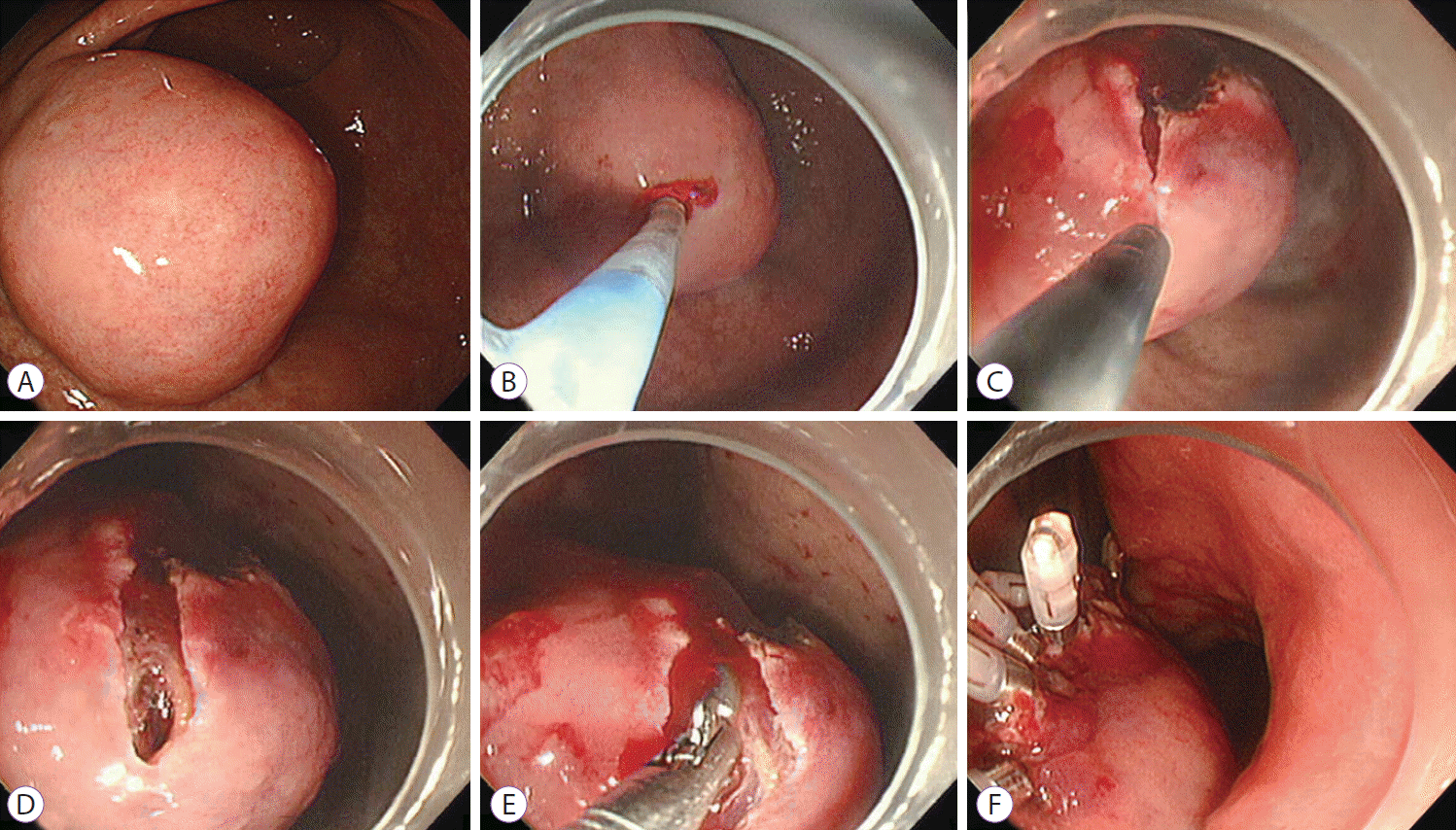
MIFB was performed as follows (Fig. 1). Indigocarmine dye in glycerol solution was injected between the SET and the mucosa. An approximately 10 mm linear mucosal incision was made at the highest convexity of the SETs by using the hook knife (Endocut mode, effect 3, output 40 W). If bleeding occurred during the mucosal incision, then epinephrine spray was applied. If hemostasis was not achieved by using the epinephrine spray, then a coagrasper was used (soft coagulation, effect 7, output 80 W). We pushed the tip of the endoscope with a transparent hood into the mucosal incision site to confirm that the tumor had been reached. When SETs were clearly exposed, a conventional biopsy forceps (FB-25K-1; Olympus Medical Systems Corp.) was introduced and tissue samples were taken. After obtaining tissue samples, the wound was closed with clips to prevent hemorrhage. On the day of the procedure, patients fasted. They were given a meal by the next morning and were discharged on day 3 after the procedure.
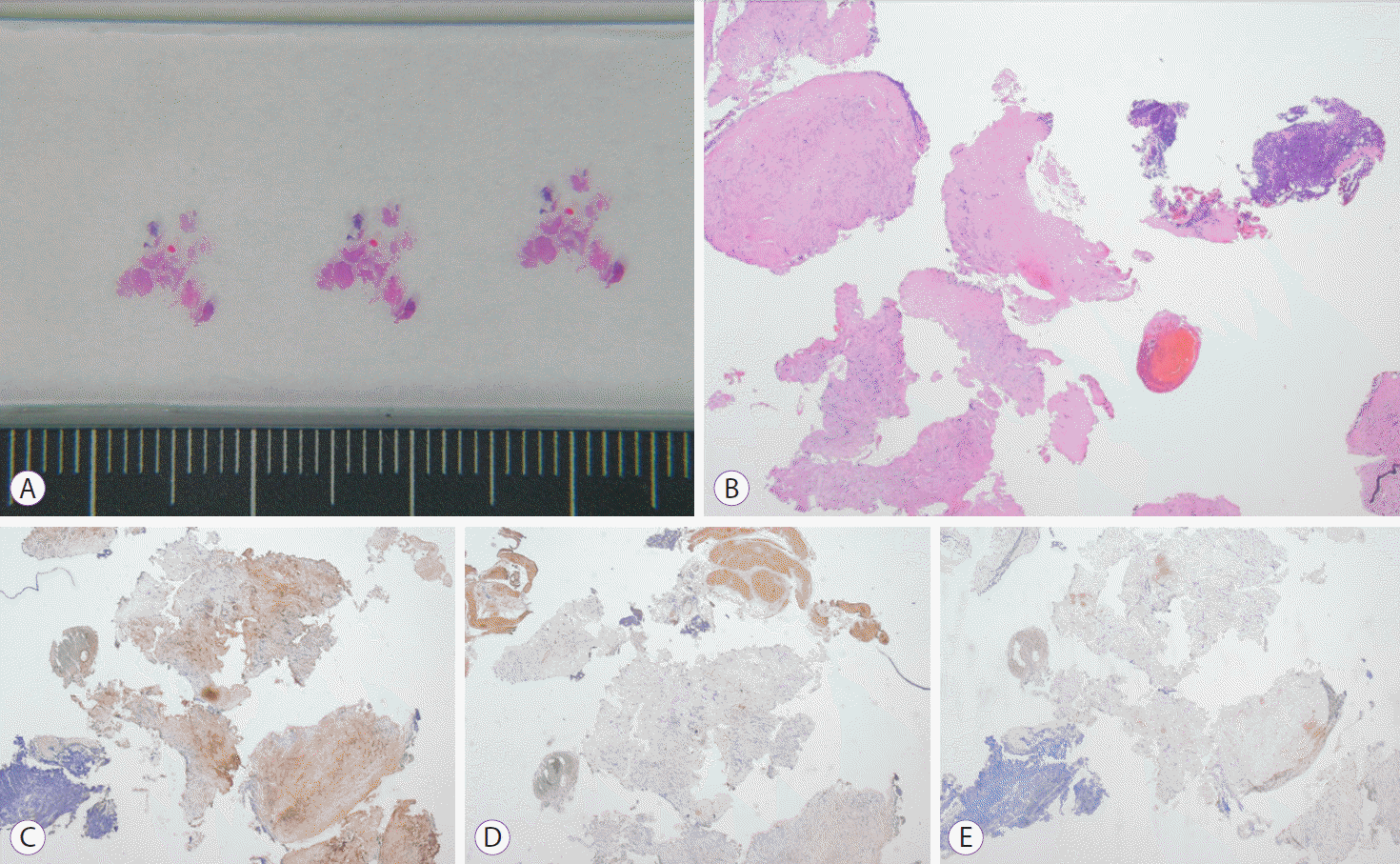
Tissue samples collected during the procedure were fixed in formalin and stained with hematoxylin and eosin. When characteristics of mesenchymal origin were noted in the hematoxylin and eosin stained specimens, specific immunochemical stains were used to differentiate between GIST and non-GIST.
RESULTS
During the study period, a total of 12 patients were enrolled (four women and eight men). The median age of the patients was 55 years (range, 27 to 75). The distribution of age was as follows: 20 to 29 years old (n=1); 30 to 39 years old (n=2); 40 to 49 years old (n=2); 50 to 59 years old (n=3); 60 to 69 years old (n=3); and 70 to 79 years old (n=1). No patients presented with symptoms related to gastric SETs. Gastric SETs were detected incidentally during endoscopic study for another purpose or through screening. Table 1 details the patient characteristics, tumor location and size, pathological diagnosis and treatment.
The median number of obtained tissue samples using MIFB was eight pieces (range, 6 to 13). Although tissue sampling was easily performed in most cases, it can be difficult in cases of schwannoma (Table 1, case 7) due to the hardness of the SET. There were no major procedure-related complications such as massive bleeding and perforation. However, during the procedure, oozing bleeding occurred at the mucosal incision site in most cases. In six cases, oozing bleeding during the procedure was controlled after spraying epinephrine. In five cases, oozing bleeding during the procedure was successfully controlled by using a coagrasper. There was no delayed bleeding.
A definitive pathological diagnosis was ascertained in 11 of the 12 cases. The pathological diagnoses among these cases included leiomyoma (n=3), lipoma (n=2), ectopic pancreas (n=2), schwannoma (n=1), and GIST (n=2). The MIFB technique was not diagnostic in one case (Table 1, case 2). For this case, we were able to obtain only eight pieces of the biopsy specimen by using MIFB. GIST was suspected on the basis of the immunohistochemical stained biopsy tissue. However, after wedge resection, it was finally diagnosed using thickened proper muscle. MIFB provided sufficient tissue samples for immunohistochemical staining for five cases of GIST and leiomyoma. In addition, it was possible to evaluate the mitotic index for all cases of GIST. Mitotic indexes of two cases of GIST were 0/50 high-power fields (HPFs) and 1/50 HPF (Table 2).
One patient with schwannoma underwent wedge resection (Fig. 2). No additional surgical resection was performed in another patient with GIST because he was classified as being at very low risk according to FletcherŌĆÖs classification (Table 1, case 1). One patient with GIST underwent wedge resection because he was classified as being at low risk.
DISCUSSION
Gastric SETs are usually encountered during routine endoscopy. Most of these lesions need follow-up endoscopy or EUS. Imaging studies such as endoscopy or EUS cannot provide exact pathological diagnoses. Therefore, the morphologic features of EUS alone have limitations for diagnosing a variety of SETs, and EUS does not provide sufficient data about whether the gastric SET is malignant or benign. Gastric SETs include a variety of pathological diagnoses. The management plan, prognosis and follow-up period may be modified according to the exact pathological diagnosis. Thus, the need for histological diagnosis for gastric SETs is likely to increase.
EUS-FNA has become the standard method for obtaining tissue samples of gastric SETs. However, the results of EUS-FNA appear to be unsatisfactory. Recently, Hoda et al. [6] reported the yield of EUS-FNA for 112 upper gastrointestinal SETs as 61.6% diagnostic, 22.3% suspicious, and 16.1% nondiagnostic with an overall accuracy rate of 83.9%. Mekky et al. [8] described the diagnostic yield of EUS-FNA for 141 gastric SETs and found that the overall results of EUS-FNA were 43.3% diagnostic, 39% suggestive, and 17.7% nondiagnostic, with an overall accuracy rate of 83%. Adequate tissue samples were obtained from 117 of 141 cases (83%). However, for 29 of the 117 cases, the samples were found to be insufficient for immunohistochemical analysis. Adequate tissue samples for histological diagnosis were obtained for only 88 of 141 cases (62%). Because immunohistochemical analysis is essential to diagnose gastric SETs that originate from mesenchymal tissue, the results of EUS-FNA were considered unsatisfactory. Therefore, there has been an interest in developing a new modality for reliable tissue sampling of gastric SETs.
Endoscopic partial resection with the unroofing technique has been suggested in this setting [10]. The overlying mucosa was removed by using a conventional snare with electrical current to expose the tumor sufficiently. Next, the exposed tumor was partially resected by snaring. Although its diagnostic yield was as high as 93.7%, blood oozing during the procedure was relatively common (56.0%), and there can be difficulties grasping gastric SETs when they have extraluminal growth. Another method using the endoscopic submucosal dissection technique was introduced [11]. A 15 mm sized round incision was made in the overlying mucosa by using flex and an IT2 knife. Next, submucosal dissection was performed with the IT2 knife and multiple endoscopic biopsy samples were taken. After the procedure, incisions were closed by clipping. In this study, pathological diagnoses were successful for all nine cases and there were no complications such as bleeding and perforation. However, this study is limited by its small study population.
In our study, endoscopic biopsies were successful in most cases. Our results are promising because sufficient tissue samples were available for a definite diagnosis. Because the size of the tissue samples using this technique is similar to that of tissue samples collected during routine endoscopic biopsy, this technique enables immunohistochemical analysis and evaluation of the mitotic index for all mesenchymal tumors. There were no complications such as major bleeding and perforation. Although minor bleeding occurred, it was easily controlled by using epinephrine spray or endoscopic electrocoagulation. Because this technique does not require expensive linear EUS and FNA equipment, MIFB can be performed at any hospital during routine endoscopy.
One limitation of this study is the small sample size. Because it was performed in a relatively small single center, this study could not be designed to have a sufficient sample size. The sample size limits the generalizability of this study. Therefore, a large, multicenter, prospective, randomized study is required to obtain more evidence confirming our results.
In conclusion, MIFB can be used as one of several methods to obtain adequate tissue samples of gastric SETs. It may serve as a preferable alternative to conventional EUS-FNA. Further investigation with a large number of patients is required to assess the safety and efficacy of this technique.