INTRODUCTION
BarrettŌĆÖs esophagus is the main risk factor for the development of esophageal adenocarcinoma. Currently, the most widely used endoscopic therapies for BarrettŌĆÖs adenocarcinoma include endoscopic mucosal resection and endoscopic submucosal dissection (ESD) [1,2], which result in complete resection. However, several investigators have reported on the occurrence of metachronous recurrence after the initial endoscopic resection [3-5]. This may occur as multiple lesions at the initial endoscopic examination, and therefore early detection of such lesions is ideal to achieve complete resection and obtain improved survival rates with minimally invasive treatment [4-6].
Narrow band imaging (NBI) is useful for the detailed examination of adenocarcinoma associated with BarrettŌĆÖs esophagus, which includes detection of the irregular microvessels of esophageal and gastric cancer at high magnification [4-6]. However, this technique has limitations, such as the difficulty to brightly view distant lesions in a large luminal organ such as the stomach.
Recently, a laser endoscopic imaging system (Fujifilm Co., Tokyo, Japan) has been developed to facilitate new diagnostic approaches for gastrointestinal lesions [7,8]. This system allows multiple modalities of endoscopic imaging by using white light laser, flexible spectral imaging color enhancement (FICE), blue laser imaging (BLI), and linked color imaging even at a distant view. Therefore, this system may be useful for the detection of adenocarcinoma associated with BarrettŌĆÖs esophagus. We report on a patient with three synchronous lesions followed by one metachronous lesion in a long segment with changes of BarrettŌĆÖs esophagus, all diagnosed with this new laser endoscopic imaging system.
CASE REPORT
A 71-year-old woman was referred for further evaluation of an uneven surface mucosa in the esophagus, in a BarrettŌĆÖs area on the anterior wall of the distal esophagus where well-differentiated adenocarcinoma was diagnosed with biopsy. The patient has a history of cerebral infarction at age 50 years, from which she recovered, except for moderate left hemiparesis. Although she also has a history of chronic heart failure, her cardiac function was improved. Her physical examination was normal; blood and serum chemistry tests including coagulation studies were normal; and chest and abdominal computed tomography scans showed no metastatic lesions. She has been routinely followed up for the development of malignant lesions in a long segment of BarrettŌĆÖs esophagus.
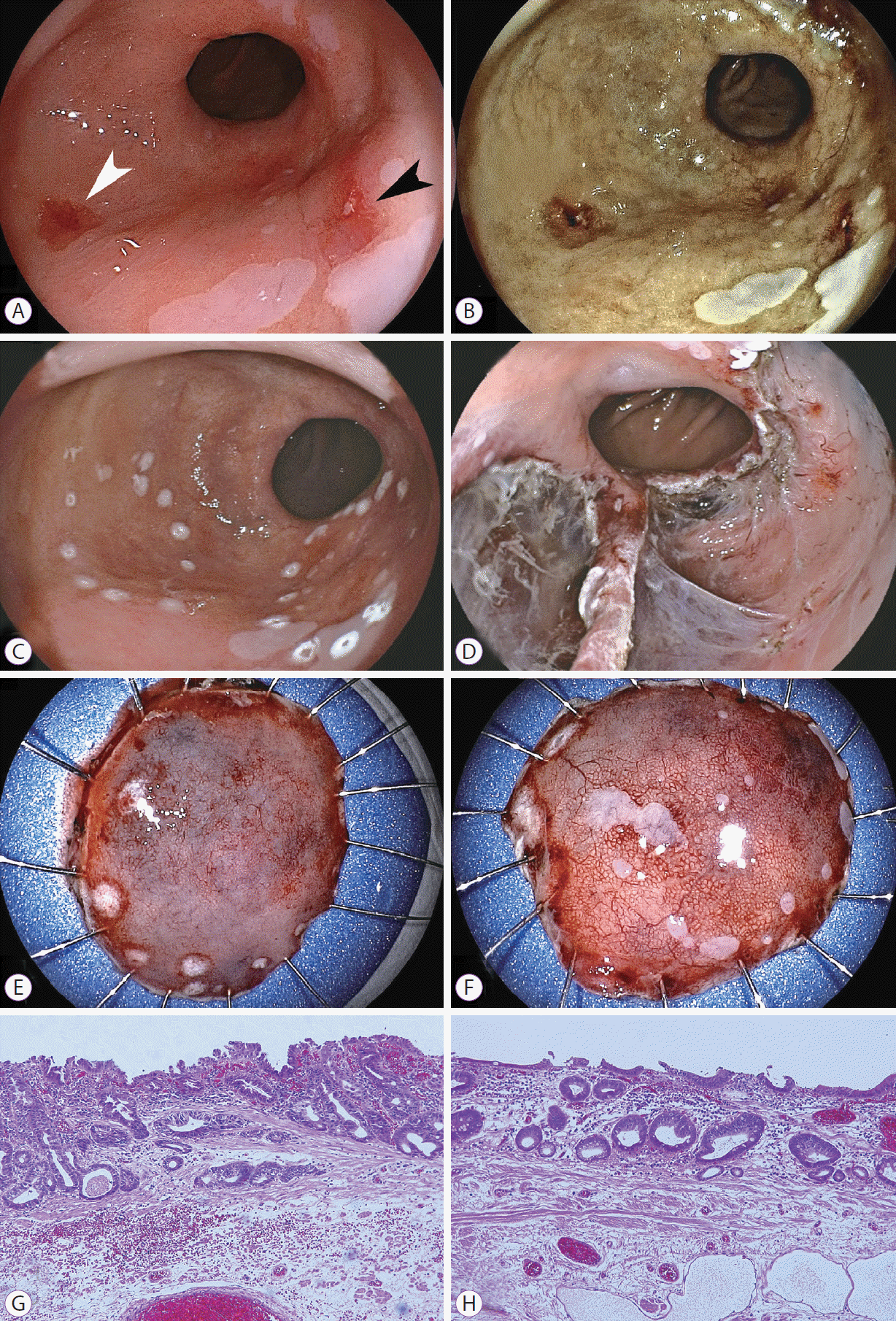
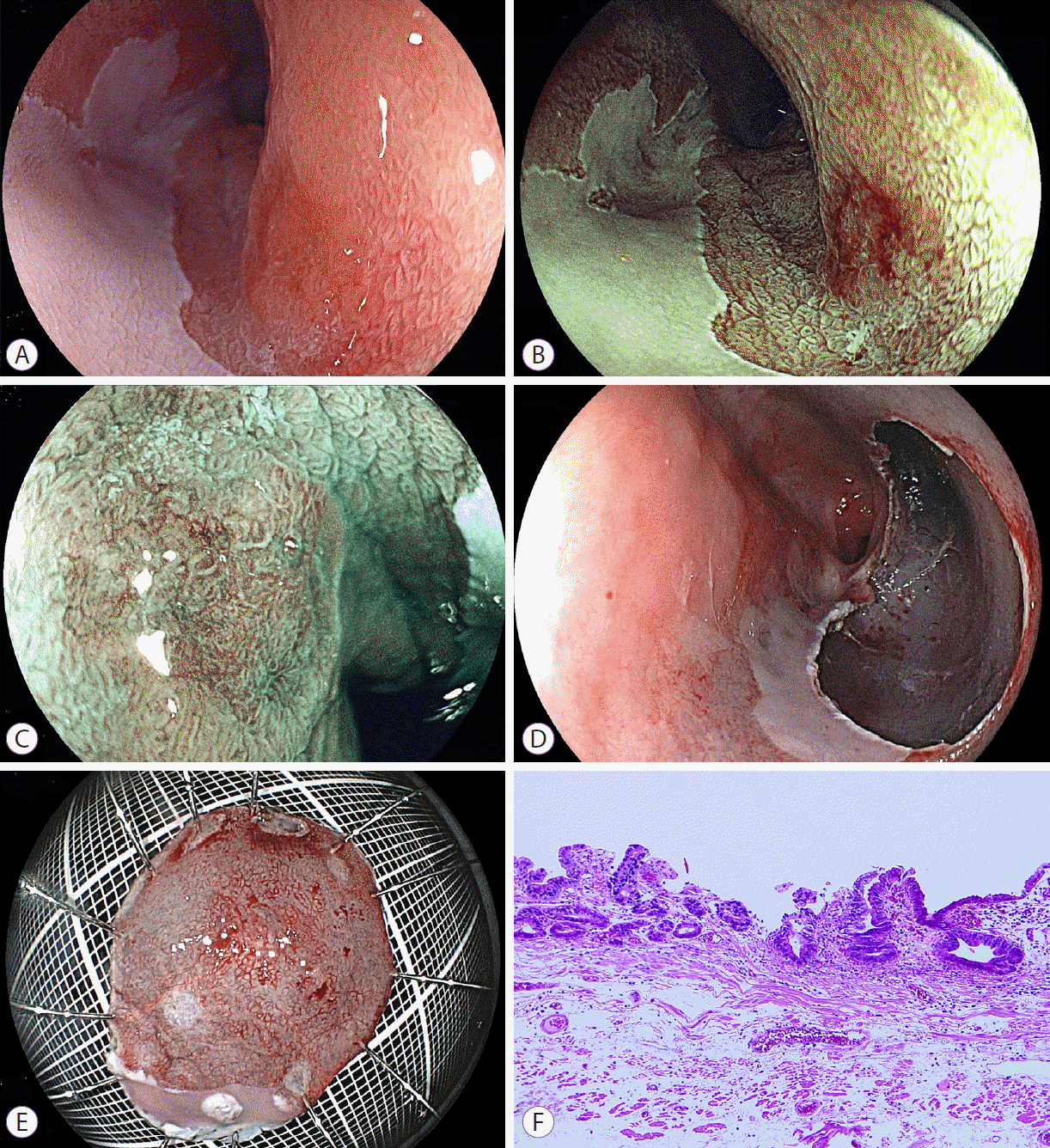
According to the Prague classification, the circumferential extent (C) of BarrettŌĆÖs esophagus was 4 cm and the maximum length (M) was 7 cm. By using a laser imaging system, we found reddish lesions on the anterior and posterior walls of the distal esophagus in white light laser images (Fig. 1). In addition, another lesion in the middle esophagus was detected as a brownish area, by using FICE and BLI (Fig. 2). All three lesions were 0-IIc early cancers with slight reddish color changes in the laser white light images: two lesions measuring 15 and 10 mm in diameter on the anterior and posterior walls of the distal esophagus, respectively, and another lesion measuring 5 mm in the middle esophagus. FICE and BLI enhanced all three lesions with high contrast, compared with the surrounding mucosa. Magnification BLI images revealed an irregular surface pattern on the brown lesion compared with the surrounding mucosa and an irregular vascular pattern in one portion of the tumor. All biopsy specimens showed atypical cells, suspicious for well-differentiated adenocarcinoma.
Three synchronous early adenocarcinomas in a long segment of BarrettŌĆÖs esophagus were diagnosed. ESD was scheduled for the two lesions in the distal esophagus at the first resection and for the lesion in the middle esophagus 3 months later.
All lesions were resected completely through ESD with sodium hyaluronate solution without difficulties. The en bloc resected mucosa measured 27 mm for the anterior wall lesion in the distal esophagus, 26 mm for the posterior wall lesion, and 25 mm for the middle esophageal lesion. The tumors were 10, 5, and 13 mm, respectively.
Pathological diagnosis of the resected specimens showed well-differentiated adenocarcinoma in all lesions (Figs. 1G, H, 2F). All tumors were located within the lamina propria mucosa with tumor-free vertical and horizontal margins. Double muscularis mucosae were seen in the area beneath the malignant cells in the resected specimens from the anterior wall of the lower esophagus and the middle esophagus. No tumor cells invaded the deep muscularis mucosae. Esophageal glands were found in the submucosa of the posterior wall of the lower esophagus. Lymphovascular invasion was not seen in the three resected specimens. These findings are all characteristic of adenocarcinoma in BarrettŌĆÖs esophagus.
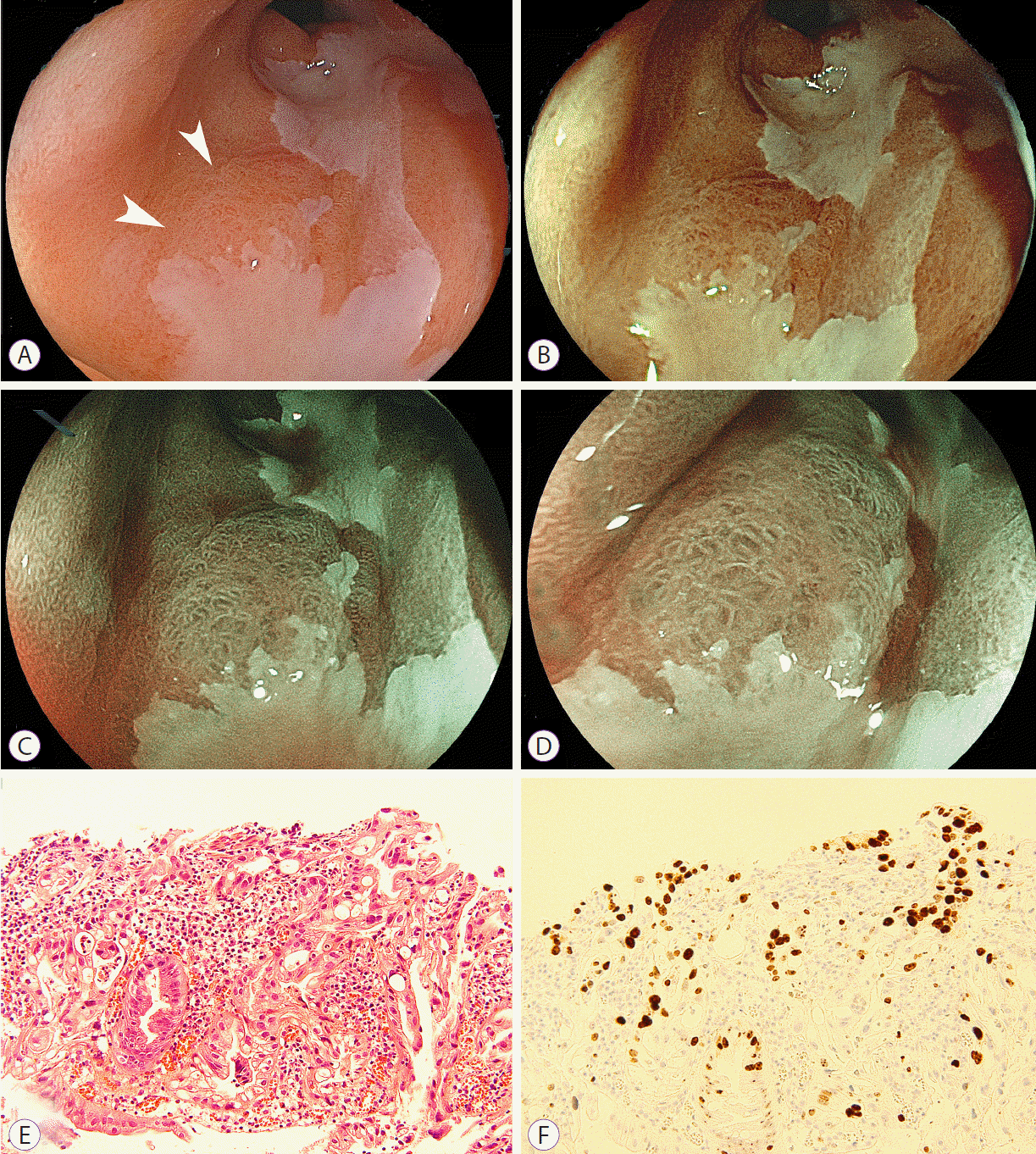
The patient was followed up with endoscopy with laser imaging every 6 months, and a fourth lesion was detected 2 years after the final ESD procedure. This new lesion had a slightly reddish area on white light laser images and an irregular surface pattern on BLI bright images (Fig. 3). Histopathology revealed well-differentiated adenocarcinoma. However, this lesion was not resected because the patientŌĆÖs cardiac function had worsened.
DISCUSSION
This report demonstrates the early detection of three synchronous and one metachronous neoplasms in a patient with a long segment of BarrettŌĆÖs esophagus, by using white light, FICE, and BLI images produced with a newly introduced laser imaging system. Three synchronous malignancies were completely resected with ESD.
Patients with a diagnosis of BarrettŌĆÖs esophagus are at a risk of developing high-grade dysplasia or esophageal adenocarcinoma. Recurrence and metachronous lesions have been shown to be the major problems with endoscopic resection in patients with early BarrettŌĆÖs neoplasia [3-5]. The prevalence of metachronous lesions or disease recurrence increases to 14% within 12 months, and to 21.5% at 5 years [3]. However, such lesions may be missed at the initial endoscopic examination. Several investigators recommend that even with high-resolution endoscopy, four-quadrant biopsies are necessary after careful evaluation of BarrettŌĆÖs mucosa to exclude synchronous neoplastic lesions [1,2]. The biopsy specimens provide histological information on the mucosa with BarrettŌĆÖs changes; however, only a small fraction of the mucosa can be sampled [9,10]. Lesions harboring dysplasia or carcinoma are easily missed [2]. The laser imaging system can produce high-resolution images even with white light imaging. Before obtaining biopsies, detailed observation by using laser imaging may also be useful for the detection of synchronous neoplasms and may compensate for the disadvantages associated with four-quadrant biopsies.
In the current patient, one missed lesion was detected by using high-resolution white light laser images. Laser endoscopic images are strongly associated with the higher emission intensity of short wavelengths illuminated by using blue laser light rather than xenon light. One of the main advantages is that the higher emission intensity of short wavelengths enables the observation of bright high-resolution images of the mucosal surface at a relatively distant view. Another missed lesion was detected as a brownish area, by using FICE and BLI. BLI and FICE images reveal malignancies with high contrast compared with the surrounding mucosa. Magnifying BLI is closely related to short wavelengths and reveals a clearly irregular microstructural pattern (Figs. 2, 3). These findings are not seen in the surrounding mucosa. It is noteworthy that these abnormal findings are obtained at relatively low magnification. However, FICE is less related to short wavelengths and cannot sufficiently demonstrate the microstructural pattern, which may be a disadvantage.
In contrast, images of the microvasculature need higher resolution than those of the microstructural pattern and a nearer view with high magnification for a more precise evaluation, even with the laser imaging system. Laser imaging is produced by using longer wavelengths unrelated to absorption by hemoglobin in addition to short wavelengths related to hemoglobin absorption. Longer wavelengths responsible for white light images are associated with imaging of the microstructure with low magnification or at a distant view, but not with the microvasculature on the tumor surface. Thus, laser imaging with low magnification can show the microstructure but does not sufficiently image the microvessels on the tumor surface.
Image-enhanced endoscopy with high magnification allows the detailed observation of the microvascular and microstructural patterns of BarrettŌĆÖs cancer. Several studies with NBI described irregular microvascular and microstructural patterns that were highly associated with high-grade dysplasia/early cancer and showed high sensitivity and specificity of NBI in detecting neoplasia [11-14]. Singh et al. [15] reported that BarrettŌĆÖs esophagus had four morphologic patterns. They described that a distorted pit with irregular microvasculature corresponds to malignant lesions. These findings are important in diagnosing BarrettŌĆÖs cancer by using image-enhanced endoscopy such as NBI and BLI.
With the laser imaging system, a recent report revealed that linked color imaging is useful for the early detection of gastric cancer [16]. In the future, linked color imaging should be evaluated for the detection of BarrettŌĆÖs adenocarcinoma.
In conclusion, laser endoscopic imaging may facilitate the detection of malignancies in patients with early BarrettŌĆÖs adenocarcinoma, similar to early gastric cancer [7]. Further assessment in a large study is necessary to evaluate the usefulness of endoscopic diagnosis for multiple adenocarcinomas in areas of BarrettŌĆÖs esophagus.