INTRODUCTION
Endoscopic submucosal dissection (ESD) is an endoscopic technique for removing neoplasms confined to the superficial layers or subepithelial tumors of the gastrointestinal tract using an en bloc resection method [1]. ESD, which is now widely used, is an important component of the treatment strategy for early gastrointestinal cancers and subepithelial tumors [2]. ESD owes its popularity to the development of newer and better instruments for the procedure. The development of newer endoscopic and accessory devices for ESD has focused primarily on reducing complications, such as bleeding and perforation, as well as shortening the procedure time. Additionally, there is a focus on the development of devices that can ensure complete resection of lesions and allow ESD to be performed for lesions located in distant and difficult-to-access areas. As the number of ESD procedures performed has increased, the number and types of accessory devices used in the procedure have also increased. Although many new devices have been developed and marketed, instruments and accessory devices used for ESD are largely determined by individual preferences and experiences. Accessory devices frequently used currently are essential tools for ESD.
The main instruments used for ESD procedures are the endoscopic electrical knife, endoscopic snare, cap, hood, injection material, electrosurgical unit, and hemostatic devices, etc [3]. Some of these devices are used not only for ESD but also for other endoscopic procedures, such as polypectomy. In this article, we review the endoscopic electrical knife, cap, hood, and hemostatic devices, which are predominantly used in ESD.
ESD KNIVES
In classic endoscopic resection prior to the introduction of ESD, an electric knife was used primarily to pre-cut the area around the lesion before using a snare for gastrointestinal lesions. During ESD, an endoscopic electric knife is an important accessory device for dissecting the submucosal layer of the lesion and the surrounding region. Various endoscopic knives are currently used clinically, with newer ones being developed. Each knife has its own characteristics, advantages, and disadvantages based on the position, shape, and characteristics of the lesion. Thus, there is no one endoscopic knife superior to all others in all respects, and it is important to select an endoscopic electric knife that is best suited for each procedure based on the location, pathological type, shape, and characteristics of the lesion to be excised. Most importantly, the gastrointestinal endoscopistŌĆÖs comfort level with a particular knife must be considered for its safe and effective use. In this article, we review various types of endoscopic electric knives that are commonly used by gastrointestinal endoscopists.
Insulation-tipped knife
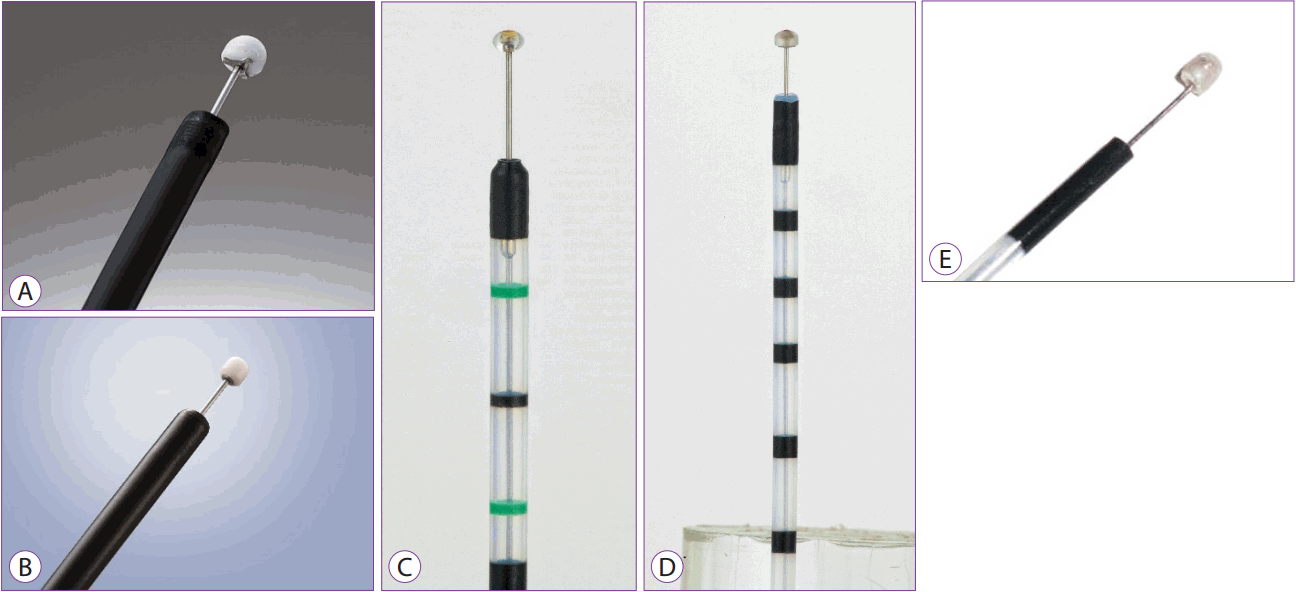
The insulation-tipped (IT) knife is a knife with an insulator at its end, measuring 2.2 mm in diameter in order to avoid puncturing the tissue. It has been widely used for ESD since its introduction by Mori et al. in the late 1990s [4]. Currently, a 4-mm long IT knife 2 (manufactured by Olympus) is in use as an improvement over the original form, and other similar knives have been developed and are also in use (Fig. 1A). The IT knife can be used for pre-cutting around the lesion and for submucosal dissection. It can also be used to achieve hemostasis in cases where bleeding is not severe. Recently, the IT knife nano is being marketed. It has a small insulation tip measuring 1.7 mm or 3.5 mm in length and is shorter than IT knife 2 (Fig. 1B). The IT knife nano allows ESD to be performed more easily and safely and has wide applications in esophageal or colorectal lesions (Fig. 1B) [5]. Currently available IT knives are the single-use IT knife (IT knife 2, KD-611L; Olympus, Tokyo, Japan), single-use IT knife nano (KD-612L; Olympus, Tokyo, Japan), Ball ESD knife (MTW, Wesel, Germany), hemispherical ESD knife (MTW, Wesel, Germany), and Helmet snare ESD knife (MTW, Wesel, Germany) (Fig. 1A-E).
When performing ESD using an IT knife, an instrument such as a needle-type knife is required to make an initial incision into the lesion. After the initial incision, the IT knife is available for pre-cutting and submucosal dissection. Because of the attached insulator, the risk of perforation is relatively low and submucosal dissection is possible even for lesions where visualization of the operative field is poor. Pre-cutting around the lesion using an IT knife is performed such that the mucosa is pulled away from the distal part of the lesion. ESD can be performed with the entire IT knife to shorten the procedure time. An additional advantage is the insulator that reduces the risk of perforation even if the lesion being dissected is not easily visualized in the operative field. Therefore, it is possible to perform resection with an IT knife, even in patients with subepithelial tumors or lesions with fibrosis [6].
Needle knife
A needle knife can be used for both pre-cutting and submucosal dissection, allowing the user to mark the area around the lesion, and has been used first in its simplest form. The length of the needle is adjustable, but the needle needs to be lengthened cautiously, particularly when the submucosal layer is dissected, to avoid tearing off the mucosal layer leading to bleeding or perforation.
Recently, with the introduction of several new endoscopic electric knives, the needle knife is mainly used to initiate the peri-lesional incision, and is not widely used for submucosal dissection.
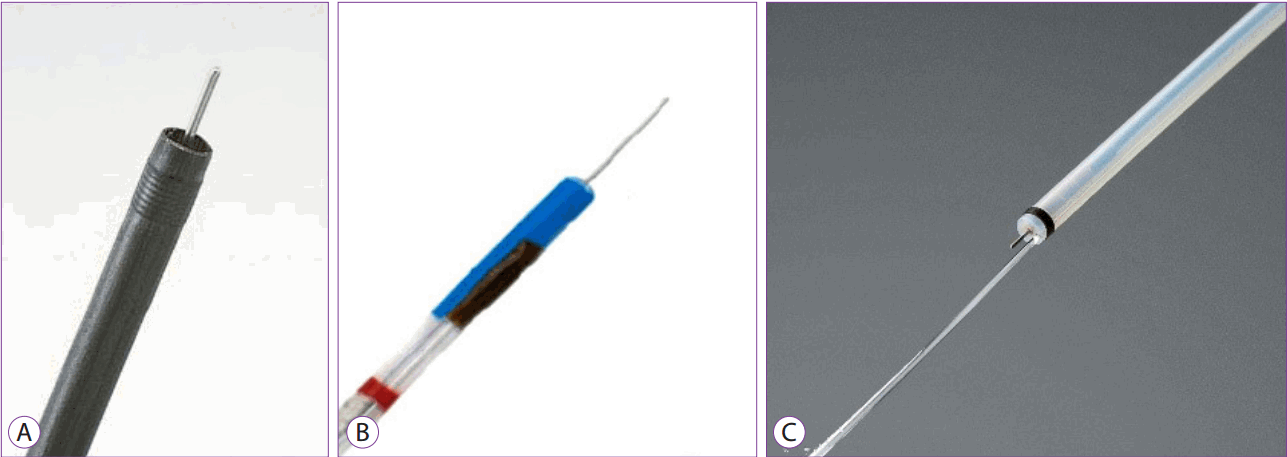
These products are manufactured by Olympus in Japan and MTW in Germany (Fig. 2A, B). The Splash knife, a PENTAX product (Fig. 2C), also uses a needle knife that performs the combined functions of submucosal solution injection and cleaning of the bleeding sites. Each ESD procedure can be performed simultaneously without the need to replace the accessories to perform different functions such as lesion marking, pre-cutting, submucosal dissection, hemostasis, injection of submucosal solution, and cleaning of bleeding sites.
Triangle-tipped knife
The triangle-tipped (TT) knife has a diathermic disc attached to the tip of a 4.5-mm long knife. It can be used for lesion marking, pre-cutting, and submucosal dissection. In cases where bleeding is not severe, coagulation can be performed for hemostasis. The TT knife is used to make a peri-lesional incision. Pre-cutting is performed by pulling the mucosa from the farthest site from the lesion. Submucosal dissection is performed by pulling the submucosa using a diathermic disc at the end of the knife.
Unlike the IT knife, the disadvantage of using a TT knife is a longer procedure time because dissection is carried out using the tip of the instrument and only a small area can be dissected at a time. There is also the risk of perforation if the area being worked upon is outside the gastrointestinal endoscopistŌĆÖs field of view. However, this knife has a definite advantage in that detailed dissection can be performed. Olympus products are now used (Fig. 3) and the TT knife is known to show good results in peroral endoscopic myotomy in cases of achalasia [7].
Flex knife
The Flex knife is a soft semicircular snare-type knife for marking, pre-cutting around the lesion, submucosal dissection, and hemostasis. It consists of a rounded snare in a plastic enclosure with excellent flexibility and operability, and it has an adjustable length.
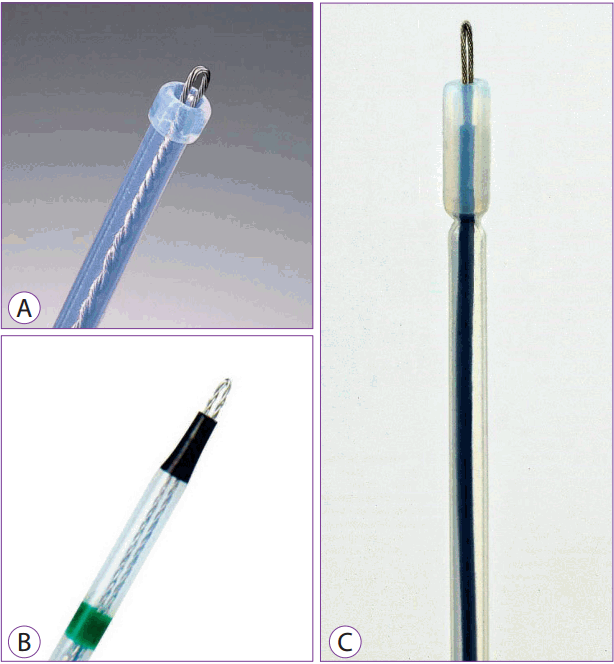
It is used to mark the periphery of the lesion, and after the lesion is incised, submucosal dissection is performed by pushing the submucosa away from the side near the operator. Because the exterior of this knife is made of flexible material, the risk of perforation is relatively small even if the knife is removed because of abrupt movement. However, as only a small area can be dissected at a time, procedure time is prolonged. Even lesions lying perpendicular to the knife can be incised and dissected in all directions because of the flexibility of material that makes up its exterior. Olympus, Kachu Technology, and MTW products are available commercially (Fig. 4A-C). The ability to wash the bleeding sites while performing other functions is an added advantage offered by a Kachu Technology product.
Dual knife
The dual knife (Olympus, Tokyo, Japan) is an instrument that incorporates the advantages of the above-mentioned flex knife and is more convenient to use (Fig. 5A). It is 2 mm long with a knob-shaped tip and an adjustable length. It cuts when its tip moves forward and coagulates the bleeding site when the knife returns to its position in front of the tip. This knife enables marking around the lesion, making an incision, pre-cutting, submucosal dissection, and hemostasis. It is also possible to cut using a rounded knife with an exterior made of plastic material to provide excellent flexibility and operability.
The dual knife is first used to mark and then incise the lesion, and after pre-cutting, submucosal dissection is carried out as if pushing the submucosal tissue away from the side near the operator. Owing to a flexible exterior, there is a relatively small risk of perforation even if the knife moves off due to sudden movement. A knife measuring 2.0 mm in length is used in the stomach, while one that is 1.5 mm in length is used in the colon. Only a small site can be dissected at a time, thus, prolonging the procedure time. Notwithstanding this disadvantage, this knife allows the operator to directly view the dissection site and cut carefully, thereby ensuring a detailed dissection. Even lesions lying perpendicular to the knife can be incised and dissected in all directions owing to the flexible surface material.
Recently, a dual knife J (Olympus, Tokyo, Japan) has been developed and marketed. In addition to the advantages of the existing dual knife, a jet can be added to the device to help with a convenient submucosal injection, such that submucosal injection can be performed along with cutting without the need to replace the device (Fig. 5B).
PENTAX has recently launched the Splash-M knife, a device similar to the dual knife with respect to shape and principle of action, for marking around the lesion, making an incision, pre-cutting, and submucosal dissection. Additionally, the incorporated water jet ensures that bleeding sites are easily visible in cases involving bleeding during ESD (Fig. 5C).
Hook knife
The hook knife has an L-shaped hook at its tip that can cut lesions horizontally and vertically. The length of the longitudinal and horizontal axis is 4.5 mm and 1.3 mm, respectively. It can be used for marking of lesions, incision, pre-cutting, and submucosal dissection. If bleeding is not severe, coagulation can be used for hemostasis. Being able to rotate the incision angle 360 degrees and to adjust the knife to a desired direction are distinct advantages of its use.
This knife is used to first mark the periphery of the lesion, and then to incise the lesion from the part further away from the operator. Dissection is begun using hooks, and the knife is aligned horizontal to the mucous membrane. During the course of dissection, the mucosa is pulled toward the operator. During dissection, the direction of the hook must be constantly adjusted based on the shape and location of the lesion being removed.
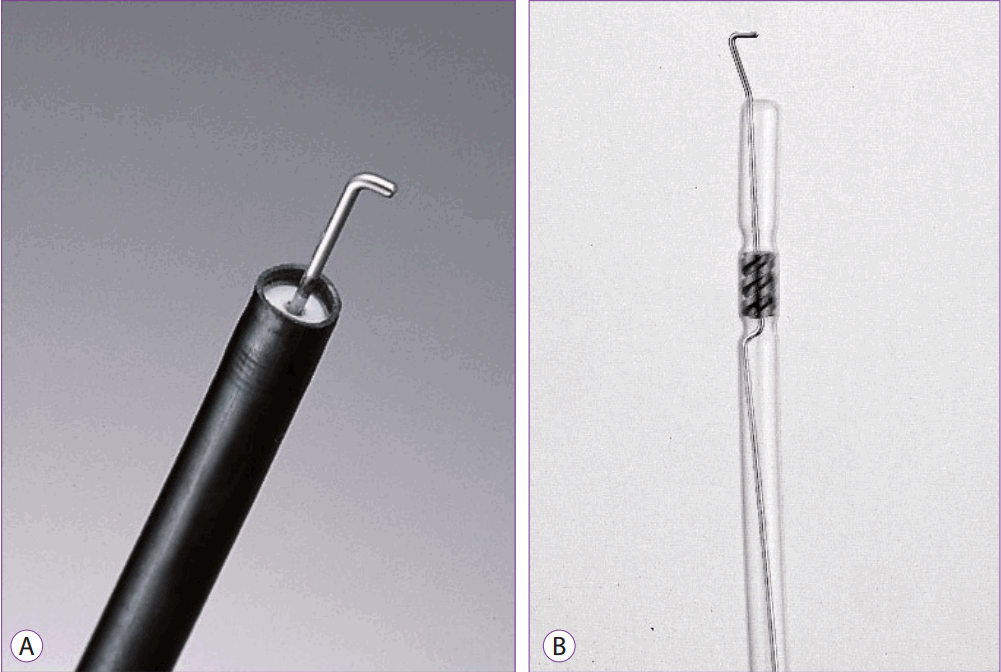
However, because only a small area can be cut at a time and the direction of the hooks must be constantly changed, procedure time is prolonged. If the site of dissection is not visible in the operative field, there is a risk of perforation; thus, most procedures are performed using a transparent cap attached. However, as the operation is performed with direct confirmation of the cut part, a more detailed resection is possible, which offers a clear advantage over other devices. Therefore, a hook knife is used in several advanced ESDs requiring detailed procedures [8,9]. Hook knives are manufactured by Olympus and MTW (Fig. 6A, B).
CAP AND HOOD
Caps attached to the end of the endoscope are very useful for the diagnosis and treatment of gastrointestinal lesions during gastrointestinal endoscopy. A transparent cap is an indispensable aid for visualizing the operative field and stabilizing the operative procedure. Currently, various types of caps and hoods are used clinically in ESD, and new caps are being developed. This article focuses on caps and hoods that are commonly used in ESD procedures.
Since traction devices have not yet been developed for use in ESD procedures, ancillary devices have become important in such procedures. The endoscopic hood helps expose the cut surface by lifting a flap after pre-cutting the lesion. If the endoscope with the hood is inserted into the submucosal layer after incising the mucosa around the lesion, the hood will effectively lift the mucosa to be incised, thereby widening the gap between the mucosal and muscle layer to provide a better view to perform submucosal dissection. Because the esophageal and colonic walls are very thin, there is a greater risk of perforation or bleeding when working in these locations if they are cut without ensuring a clear view of the submucosal and muscle layer. Thus, a cap and hood are helpful in providing good visibility during operations in these areas.
The endoscopic hood limits accidents by reducing movement of lesions caused by peristalsis in the stomach or intestines, respiration, and heartbeat. Small and precise movements of the endoscope require that a certain distance be maintained between the endoscope and the lesion. A hood attached to the endoscope is very useful for this purpose. When using the hood, the operator lightly presses the mucosal and submucosal layer with the tip of the hood to secure the field of view, and proceeds with dissection after adjusting the length of the knife needle at the tip of the hood. In addition, the hood can identify the submucosal blood vessels and sites of hemorrhage on the endoscopic screen while maintaining a certain distance from the endoscopic lens, so that the target blood vessel can be accurately gripped and coagulated using a hemostatic device.
Existing hoods include Olympus hoods or a small caliber-tip transparent hood (ST hood; Fujinon, Tokyo, Japan), and a hood that sprays saline at high pressure (Endo irrigation cap; Mesa Medical, Seoul, Korea; Fig. 7).
In previously used endoscopic hoods, blood or water would often collect on the inner surface of the hood attached to the end of the endoscope during ESD procedures and interfere with the field of view. Previously available hoods could not prevent this occurrence because they had no side hole, but new hoods have been fitted with a side hole to overcome this disadvantage. The method of mounting the hood depends on the procedure adopted by the gastrointestinal endoscopic surgeon. To attach a hood with a side hole to the endoscope, the side hole of the hood must be mounted in alignment with the objective lens of the endoscope. When fluid pooled in the hood blocks the endoscopic field of view, the side hole of the attachment is brought into contact with the tissue, and this action expels the fluid.
HEMOSTATIC DEVICES
Bleeding and gastrointestinal perforation are major complications associated with ESD. Because ESD procedures require peri-lesional mucosal cuts and submucosal dissection, there is a high risk of bleeding during or after the procedure. Additionally, frequently attending to hemostasis during dissection and lesion removal lengthens the procedure time, and the operator needs to be knowledgeable about bleeding control techniques and accessory hemostatic equipment used for ESD procedures.
Coagulator probe
Electrocoagulation generates heat due to the resistance offered by the tissue when a high frequency current flows through it. The heat coagulates the tissue, thereby achieving hemostasis. Unipolar electrocoagulation is being increasingly used for hemostasis. Contact thermocoagulation using a coagulation probe is effective for hemostasis in moderate-sized arteries less than 2 mm in diameter. The high frequency current causing compression of the vessel wall suppresses blood flow through it and coagulates the blood vessel (Fig. 8A). If excessive coagulation occurs, reaching deep into the tissue, it can damage the gastrointestinal muscular layer, such that when the device is detached from the tissue, some tissue may fall off and produce profuse bleeding. The Injection Gold Probe from Boston is a bipolar hemostasis catheter with a needle that injects vasoconstrictor saline around the vessel (Fig. 8B).
Hemostatic forceps
Hemostatic forceps are shaped like hot biopsy forceps, but the contact surface of the cup is widened to distribute the current locally, resulting in hemostasis due to protein denaturation, but without burning the tissue. Caution is necessary in some areas of the gastrointestinal tract such as the colon or esophagus, which may develop delayed perforation due to coagulation. This device is useful for controlling pulsatile bleeding in the small blood vessels of the gastrointestinal tract because it can effectively achieve hemostasis by gripping and releasing electric energy to the bleeding points. Additionally, it coagulates the exposed blood vessels during submucosal dissection, even before bleeding occurs. In the event of hemorrhage associated with fibrosis, it is often difficult to obtain a sufficient hemostatic effect because gripping the connective tissue around the point of hemorrhage is challenging. In such instances, the hemostatic forceps is useful for gripping blood vessels around the fibrotic tissue.
It is important to accurately identify and properly grasp bleeders using the hemostatic forceps, by appropriately rotating the tip of the instrument. Correctly grasping the bleeding site with hemostatic forceps helps stop active bleeding. A slight pull on the tissue with the hemostatic forceps along with passage of a current through it for 1ŌĆō2 seconds will lead to a change in the color of the tissue due to protein denaturation, thereby confirming the hemostatic effect. Opening the hemostatic forceps confirms whether cessation of bleeding has occurred, and this can be further confirmed by ensuring that hemostasis is maintained even after the distension of the gastrointestinal wall with the air supply.
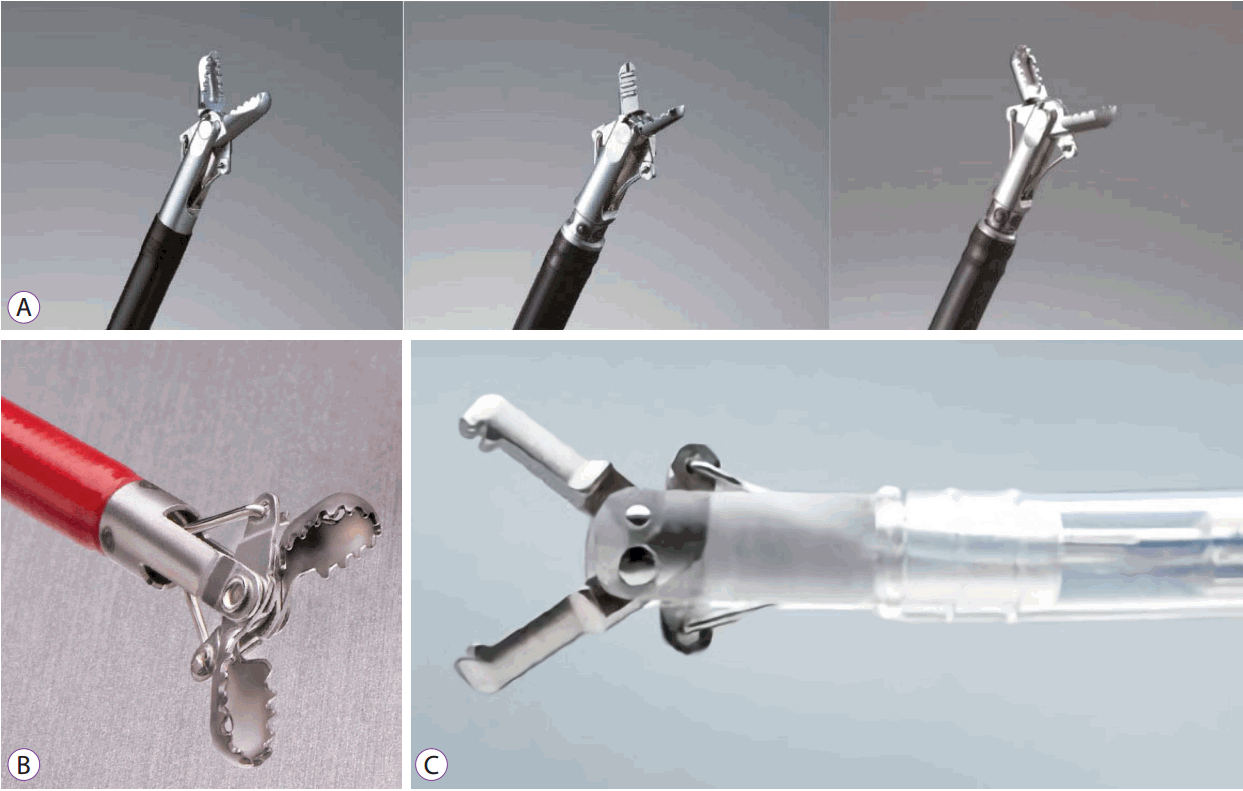
Hemostatic forceps currently available commercially include Coagrasper (monopolar, Olympus, Tokyo, Japan) and Hemostat-Y (bipolar, PENTAX, Tokyo, Japan), among others (Fig. 9A, C). The hot biopsy forceps, a product of Boston Scientific, is also available for controlling bleeding associated with ESD (Fig. 9B). While there is no difference between the two in terms of the working principle, the width of the forceps, the shape of its tip, and the presence or absence of rotatory function differ.
Argon plasma coagulation
Argon plasma coagulation is a noncontact hemostasis method used for thermocoagulation without direct contact of electrodes. The argon plasma produced by the ionization of the inert argon gas in an electric field generated by a high-frequency current enables high-frequency electrical energy to be transmitted to the tissue to effect hemostasis.
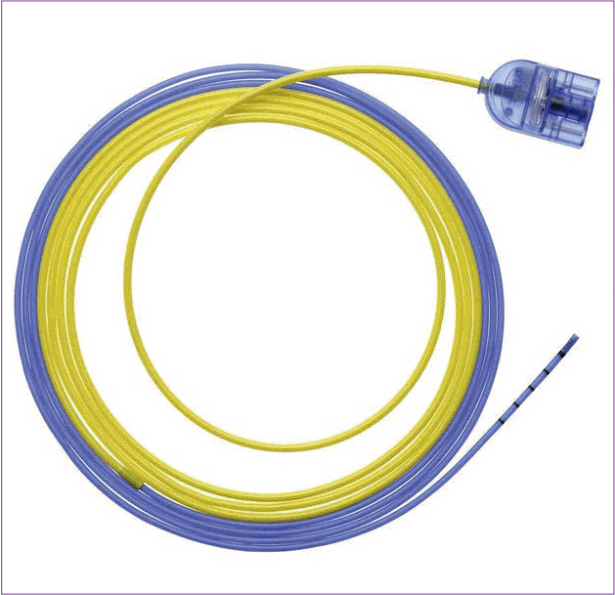
Argon plasma coagulation consists of a high-frequency source of current, a source of argon gas, a foot switch, and a probe. The probe has a unipolar electrode inside and an outer surface electrically insulated with Teflon. A flexible catheter 2.3 to 3.2 mm in diameter is first inserted into the working channel of the endoscope. Pressing the foot switch activates the high frequency current and activates the source of argon gas. The airflow of argon gas can be adjusted between 0.5 and 2.5 L/min, and the gap between the lesion and the probe is maintained between 2 and 10 mm. The stream of argon plasma dehydrates the tissue by blocking the flow of blood into the lesion with constant pressure and drying the area around the lesion. In addition, oxygen is removed from the site of the lesion and inflow of oxygen is blocked to maintain a constant temperature, thereby restricting and maintaining a constant depth of tissue, which the coagulation effect penetrates, thereby preventing excessive tissue damage.
Because the argon plasma coagulation probe does not directly contact the tissue, there is no risk of re-bleeding due to detachment of the probe after hemostasis. Additionally, when the probe approaches the lesion, coagulation cauterization can be performed not only on the front but also on the side of the catheter. ERBE and S├Čring products from Germany are commercially available (Fig. 10). In addition to hemostasis, the argon plasma coating can ablate the desired site or abdominal tissue to reduce recurrence after resection of large tumors. It also finds application in ablation therapy to remove tumors without endoscopic resection of lesions in patients with a bleeding tendency.
Clip
Clipping was a method representative of mechanical hemostasis. In 1975, Hayashi et al. first reported the usefulness of endoscopic hemostasis using metal hemostasis clips [10]. The endoscopic clip, which is widely used today, was first produced by Olympus in 1995, with several subsequent improvements in design and function [11].
Clips are an ideal hemostatic device that do not cause tissue damage or inflammation and provide an effective hemostatic effect with a low risk of perforation. However, successful clipping to achieve hemostasis depends upon the location and characteristics of the lesion and the proficiency of the endoscopist and assistant. Clipping is difficult when using a horizontal approach alone or while reversing the endoscope, and when used at the bottom of fibrotic ulcers. In addition, when hemostasis is needed during ESD, hemostasis using a clip should be avoided as it may interfere with resection or submucosal dissection. However, in cases of severe hemorrhage not controlled with other hemostatic methods such as hemostatic forceps, clipping can be considered. It should be remembered that bleeding points may not be reliably exposed when bleeding occurs through tiny gaps in the exposed white muscle layer after resection of a lesion. In such cases, hemostatic forceps are often ineffective, and excessive coagulation of the muscle layer may result in perforation. In such instances, mechanical hemostasis using a clip may be a safe option.
The clip mechanism comprises a clip and a disposable and reusable clip-fixing device. The clip has unique features that vary with the design and the manufacturer.
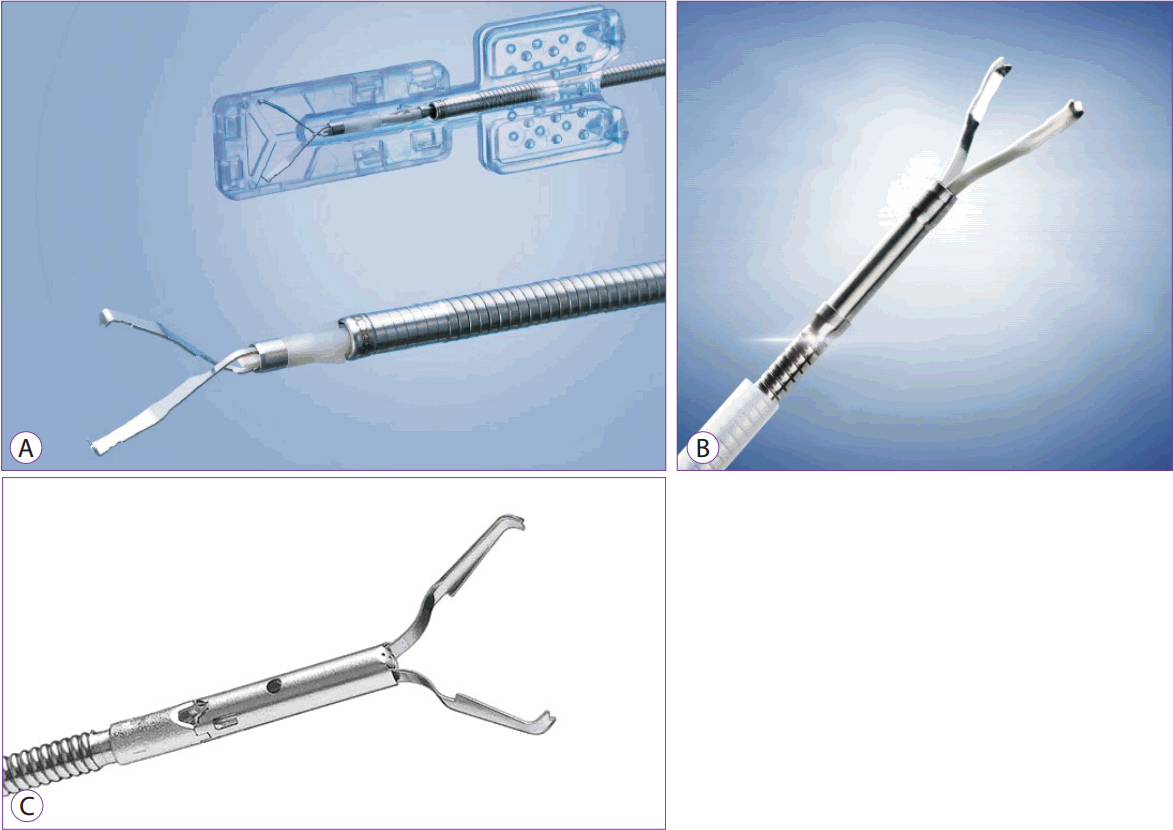
There are a variety of endoscopic clips, including a rotatable clip (EZ clip, Quick Clip 2, Quick 2 long, multi clip), a product that can open and close for accurate positioning (QuickClip ProŌäó; Olympus, Tokyo, Japan), a product that can be opened and closed again after grasping (Resolution clip; Boston scientific, Marlborough, MA, USA). There are other products that can clip multiple sites at once without reloading the clip (Fig. 11A-C).
CONCLUSIONS
Endoscopic electric knives, caps, hoods, and hemostatic tools, reviewed in this article, are key components of ESD procedures and are among the many factors that determine the success of ESD procedures. Each device is becoming increasingly popular and have become more functional and user friendly resulting in a reduced incidence of complications. Moreover, efforts are being made to improve their ease of use and efficiency. Previously, the endoscopic electric knife was used only for its cutting function. However, in recent years, many knives with various added functions and features, such as submucosal injection and water jet have been introduced.
The current instruments have their own characteristics, advantages, and disadvantages, and one instrument is not superior to others in all respects. Therefore, a gastrointestinal endoscopist must select the instrument most appropriate for a particular procedure, considering the ease of operation and safety. The development of innovative endoscopes and endoscopic accessory devices should augment and expand the scope of minimally invasive endoscopic procedures. Thus, while previously, the role of the gastrointestinal endoscopist centered on developing comfortable endoscopic techniques for patients and physicians, the focus today lies on the development of instruments to promote better techniques; thus, expanding the role of doctors.