Introduction
The incidence of colon cancer has been increasing as the society has become more industrialized [1]. In Korea, according to the National Cancer Information Center statistics for 2015, colon cancer has the second highest incidence among all cancer types.
Venous tumor thrombosis occasionally accompanies renal cancer, liver cancer, and pancreatic cancer [2]. In general, hepatic metastases frequently occur in colon cancer. However, colorectal cancer without hepatic metastases is very rarely accompanied by venous tumor thrombosis in locations such as the portal vein, superior mesenteric vein (SMV), and inferior mesenteric vein [3].
Recently, advanced imaging equipment and techniques, such as dynamic computed tomography (CT) and positron emission tomography (PET)-CT, have been developed. Following these technological advances, rare cases of venous tumor thrombosis associated with colon cancer have been reported [4,5]. We report a case of a venous tumor thrombosis associated with ascending colon cancer, and present a review of the literature.
CASE REPORT
A 46-year-old man presented to our hospital with a complaint of bloody stool. The patient, who periodically donated blood to the hospital up to two years ago, was healthy. Fifty days prior to his hospital visit, he had symptoms of abdominal pain and diarrhea related to a diet of cabbage. He visited a local hospital because of the symptoms and was diagnosed with severe anemia, with a hemoglobin level of 5.2 mg/dL. Subsequently, he was transferred to our hospital for further evaluation.
He denied previous operations or a history of diabetes mellitus, hypertension, hepatitis, or tuberculosis. Physical examination revealed no specific findings.
Laboratory results were as follows: white blood cell count 2.8├Ś103/╬╝L, hemoglobin level 4.8 g/dL, platelet count 347├Ś103/╬╝L, AST/ALT levels 11/5 IU/L, plasma sodium level 139.0 mEq/L, plasma potassium level 8.4 mEq/L, plasma chloride level 102 mEq/L, blood urea nitrogen level 12.8 mg/dL, creatinine level 1.19 mg/dL, iron level 10 ╬╝g/dL, total iron binding capacity 365 ╬╝g/dL, and microcytic hypochromic erythrocytes in peripheral blood smear. The levels of tumor markers, such as alpha fetoprotein (2.2 IU/mL) and carcinoembryonic antigen (1.7 ng/mL), were all within normal range.
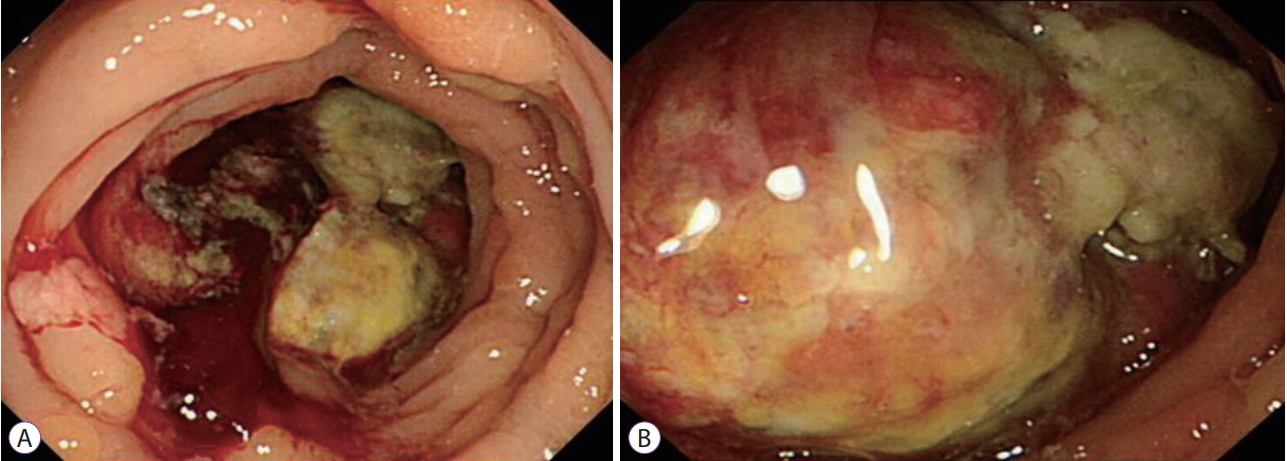
Colonoscopy revealed a protruding mass in the ascending colon (72 cm from the anal verge) that almost obstructed the lumen. The endoscope was unable to pass the lesion (Fig. 1).
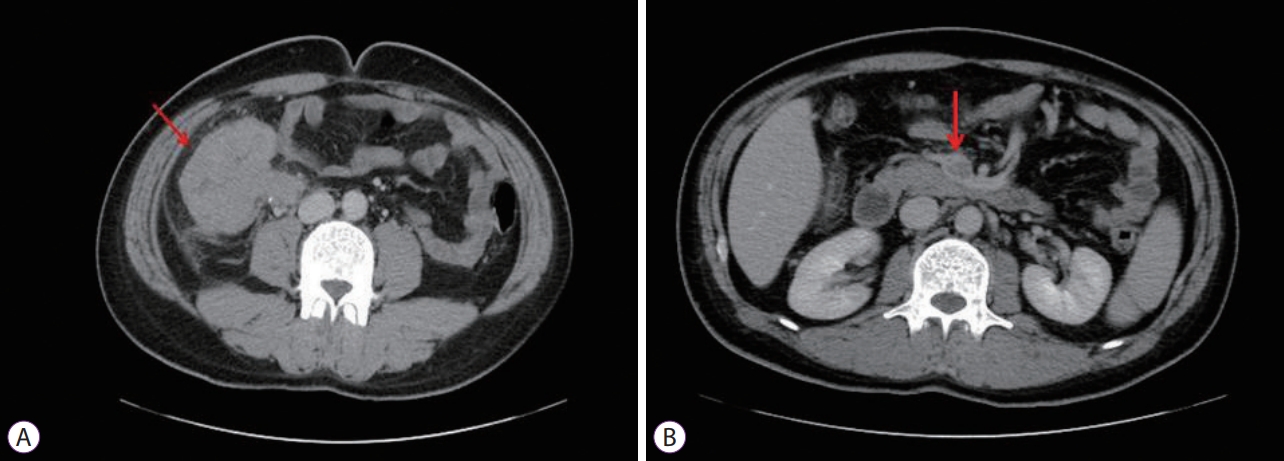
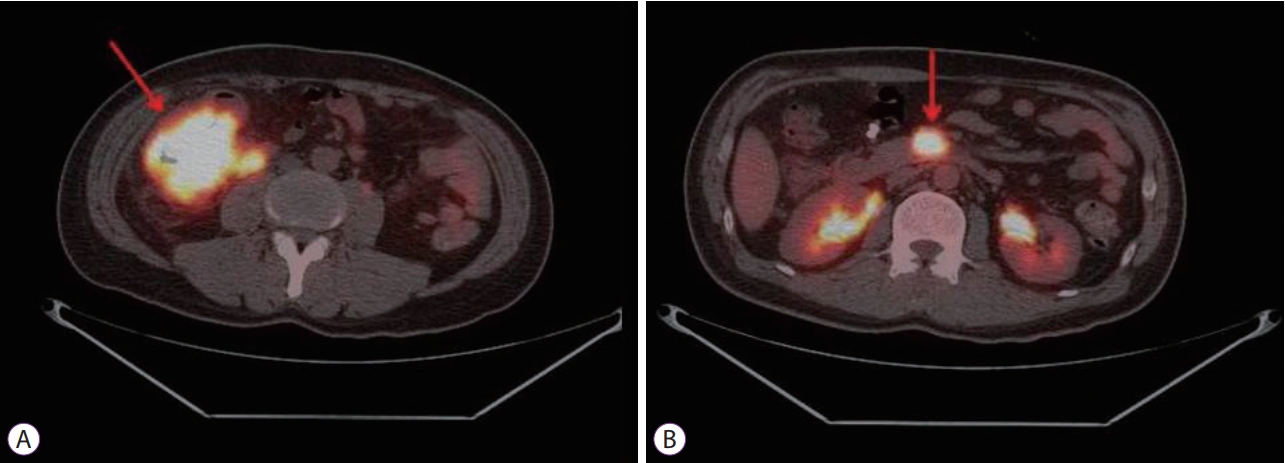
Plain abdominal radiography revealed no particular findings. Abdominal CT revealed a tumor measuring 7.5 cm in diameter in the proximal part of the ascending colon, involving the terminal ileum (Fig. 2). An intensely hypermetabolic mass (standardized uptake value [SUV] 24.7) involving the cecum and terminal ileum was noted on PET. In addition, a hypermetabolic SMV thrombus (SUV 15.8) was also noted (Fig. 3).
Patient underwent right hemicolectomy with SMV thrombectomy. After the right colon resection, the ileum and transverse colon were side-to-side anastomosed. The SMV was exfoliated and incised (5-cm incision) for thrombus removal by the vascular surgeon. After removing multiple thrombi, the site was closed with a prolene suture.
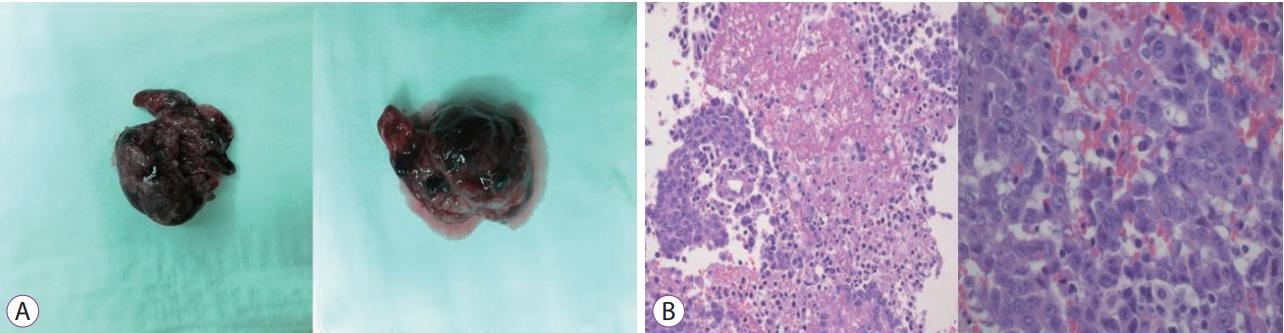
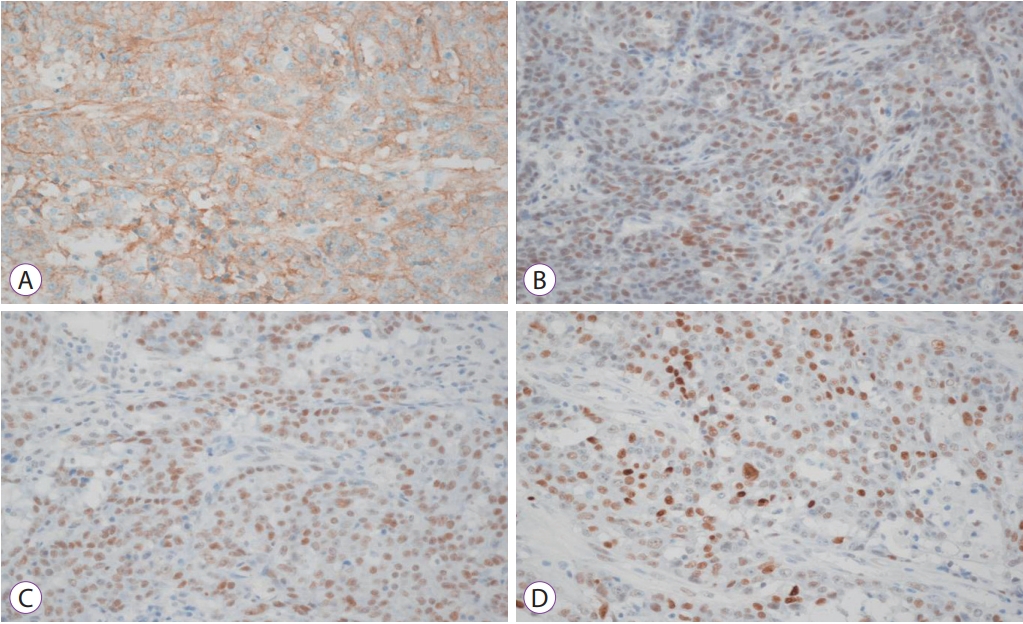
Multiple thrombi were removed. The size of lesion was variable, measuring up to 6.5 cm in greatest dimension. The thrombus was a black colored mass with granular surface (Fig. 4). The thrombus consisted of fibrinous material with metastatic adenocarcinoma cells in the background (Fig. 4). The main colon mass was a 9.5├Ś6├Ś3 cm3 poorly differentiated adenocarcinoma, located in the ascending colon, cecum, and terminal ileum. Lymphovascular and perineural invasion were observed. The immunohistochemical stain of the main colon mass showed positivity in EGFR, MLH1, MSH2 and p53 stain (Fig. 5). Pelvic wall invasion was confirmed. Sixteen of 49 regional lymph nodes were invaded by tumor cells. Therefore, the T4bN2b pathological stage was diagnosed.
After surgery, patient underwent 12 cycles of adjuvant chemotherapy (AVASTIN/FOLFIRI), and there has been no recurrence for 12 months.
Discussion
In about 15% of patients with colorectal cancer, the cancer has already metastasized to other organs at the time of cancer diagnosis, and the most common site of metastasis is the liver [6]. However, the reported incidence of macroscopic tumor thrombosis is very low, at about 2.8% [7]. In this case, an ascending colon cancer with tumor thrombus in SMV was found. The thrombus was pathologically confirmed as metastatic colon cancer.
During the diagnostic workup, CT and magnetic resonance imaging are useful to detect tumor thrombosis. Venous tumor thrombosis is appears on CT as a low-attenuation area. Similarly, blood clot thrombosis appears as low-attenuation area. Therefore, it is very difficult to differentiate between tumor thrombosis and blood clot thrombosis on the basis of CT data alone. Currently, PET is very useful in detecting venous tumor thrombosis through intense radiotracer accumulation. PET is helpful to distinguish tumor thrombosis from blood clot thrombosis [8].
Regarding treatment, complete resection of primary tumor and venous tumor thrombosis is important. According to the results of a recent meta-analysis by Otani et al., complete tumor resection was possible in 32 (74.4%) of 43 patients with colon cancer and venous tumor thrombosis [9]. In two patients, the main tumor with venous thrombosis was removed, but hepatic metastases were not resected. Surgical resection could not be performed in four patients, and the treatment was not obvious in five patients. In addition, adjuvant chemotherapy was performed in 21 (65.6%) of 32 patients [9]. In this case, primary tumor resection was performed completely. However, the vein with tumor thrombus could not be excised, because the vein was essential for the major part of small bowel circulation. Therefore, we incised the vein, removed tumor thrombus, and closed the vein with a prolene suture.
The prognosis of patients with venous tumor thrombosis related to a colon cancer is uncertain. Because venous tumor thrombosis may be an aggressive cancer, the prognosis may be poor. However, following complete surgical resection associated with adjuvant chemotherapy, some of patients show a long-term survival. According to the results of the same meta-analysis by Otani et al., 11 of 43 patients survived for more than 2 years [9]. The mean survival time was 22.5 months. This survival is not poor compared to that of stage IV colon cancer [9]. A long-term survival can be expected in patients with complete tumor resection and adjuvant chemotherapy.
In conclusion, we report an unusual case of ascending colon cancer associated with SMV tumor thrombosis. The tumor thrombosis was pathologically confirmed as metastatic colon cancer and was treated by right hemicolectomy and SMV thrombectomy.
Our case shows that patients with colorectal cancer can develop venous tumor thrombosis and warns clinicians not to overlook venous tumor thrombosis. According to the results of a meta-analysis by Otani et al., the median age of patients with colorectal cancer and venous thrombosis was 67 years (range, 47ŌĆō84 years), the ascending colon and rectum was the most frequently affected site, and more than half of patients had moderately differentiated adenocarcinoma [9]. Venous tumor thrombosis should be considered in patients with colon cancer that have these features.