Self-expandable metallic stent-induced esophagorespiratory fistulas in patients with advanced esophageal cancer
Article information
Abstract
Background/Aims
Self-expandable metallic stents (SEMSs) are widely adopted for the palliation of dysphagia in patients with malignant esophageal strictures. An important adverse event is the development of SEMS-induced esophagorespiratory fistulas (SEMS-ERFs). This study aimed to assess the risk factors related to the development of SEMS-ERF after SEMS placement in patients with esophageal cancer.
Methods
This retrospective study was performed at the Instituto do Cancer do Estado de São Paulo. All patients with malignant esophageal strictures who underwent esophageal SEMS placement between 2009 and 2019 were included in the study.
Results
Of the 335 patients, 37 (11.0%) developed SEMS-ERF, with a median time of 129 days after SEMS placement. Stent flare of 28 mm (hazard ratio [HR], 2.05; 95% confidence interval [CI], 1.15–5.51; p=0.02) and post-stent chemotherapy (HR, 2.0; 95% CI, 1.01–4.00; p=0.05) were associated with an increased risk of developing SEMS-ERF, while lower-third tumors were a protective factor (HR, 0.5; 95% CI, 0.26–0.85; p=0.01). No difference was observed in overall survival.
Conclusions
The incidence of SEMS-ERFs was 11%, with a median time of 129 days after SEMS placement. Post-stent chemotherapy and a 28 mm stent flare were associated with a higher risk of SEMS-ERF.
INTRODUCTION
Esophageal cancer is currently the eighth most common cancer worldwide, with an increasing incidence, particularly adenocarcinoma.1 Unfortunately, more than half of the patients have inoperable tumors at diagnosis, requiring other treatment options, such as chemotherapy and/or radiotherapy (RT). Self-expandable metal stents (SEMSs) are widely used in such cases.2
Although esophageal SEMS are safe, adverse events (AEs) are common, especially when stents remain in place for a longer duration, and occur in patients with longer survival.3 Esophagorespiratory fistula (ERF) is one of the most feared AEs, with a reported prevalence of 9%–10%.2,4 SEMS-induced ERF (SEMS-ERF) has a major impact on the quality of life, morbidity, and mortality of these patients as it may lead to pulmonary sepsis and death.5
To date, data regarding SEMS-ERFs are scarce. A retrospective study including patients with benign and malignant diseases who underwent SEMS placement reported a higher risk of SEMS-ERF in patients with benign anastomotic strictures, radiation therapy, and a higher comorbidity score index, with a 30-day mortality of >50%.6 A study that specifically addressed the risk factors for SEMS-ERF and its outcomes in patients with malignant diseases is lacking.
This study aimed to assess the risk factors for the development of SEMS-ERF after esophageal SEMS placement in patients with malignant esophageal strictures.
METHODS
Study population
This retrospective study was performed at the Instituto do Cancer do Estado de Sao Paulo, Brazil. All patients with malignant esophageal strictures who underwent esophageal SEMS placement between 2009 and 2019 were eligible. The inclusion criteria were patients with inoperable stage IV esophageal or extra-esophageal cancer receiving esophageal stent placement for the palliation of dysphagia or malignant esophageal fistulas. Exclusion criteria were previous esophagectomy and benign esophageal strictures.
SEMS placement
For stent placement, esophagogastroduodenoscopy (EGD) was performed in the supine position under general anesthesia using a standard forward-view endoscope (GIFH-180; Olympus America). A guidewire was placed in the stomach and the stent delivery system was inserted over the guidewire under fluoroscopic guidance. Stents were deployed under fluoroscopic guidance, and direct endoscopic visualization was performed for stents placed at the proximal esophagus to ensure a proper distance from the cricopharyngeal muscle. When a malignant fistula was present, an esophagogram was obtained after stent deployment to confirm the fistula sealing.
The length of the stent was selected to cover 2 cm above and 2 cm below the tumor. Stents with a cervical profile were preferred for lesions located in the proximal esophagus (Choostent; MI Tech) but were not used uniformly. For lesions in the middle esophagus, we used partially or fully covered stents with a body diameter of 18 to 23 mm (Wallflex and Ultraflex, Boston Scientific; Evolution, Cook Medical; Hanarostent, MI Tech; Endoflex, Voerde). For distal lesions with involvement of the gastroesophageal junction, we preferred to place partially covered stents with anti-reflux valves (Hanarostent Esophagus Valve; MI Tech), although not uniformly.
Resumption of oral liquid intake was allowed on the day after the procedure, and progression to semi-solid and solid foods was performed according to patient tolerance. Follow-up visits were conducted at regular intervals until the patient died.
Assessment of clinical outcomes
Information regarding sex, age, histological type of lesion, tumor location, Eastern Cooperative Oncology Group performance status, previous fistula, indication for stent placement, technical success, time to occurrence of SEMS-ERF, time to death, treatment with chemotherapy and/or RT, stent type, model, and size were collected.
SEMS-ERF was suspected when patients presented with symptoms of coughing after liquid intake and/or repeated pulmonary infections. The diagnosis was confirmed by one or more of the following examinations: esophagography, computed tomography, EGD, or bronchoscopy (Fig. 1).
Statistical analysis
Quantitative variables were described by calculating the measures of central tendency and dispersion. For categorical variables, absolute and relative frequencies were used. When necessary, quantitative variables were categorized based on the percentiles of the distribution of values (the 25th, 50th, and 75th percentiles). To verify the association between categorical variables, we used the chi-squared test or Fisher exact test. Hazard ratios (HRs) and 95% confidence intervals (CIs) were calculated using the Cox proportional hazard model. Mortality was adopted for competing risk analysis. Survival rates were analyzed using the Kaplan-Meier method, and the curves were compared using the log-rank test. For all analyses, an alpha error of 5% was used. Results were considered statistically significant at p<0.05.
Ethical statements
Informed consent was obtained from all the patients or their family members before stent placement. This study was approved by the Ethics and Research Committee of the Instituto do Cancer do Estado de São Paulo (registration number: NP038/14).
RESULTS
Of the 342 patients who underwent esophageal SEMS placement, seven were excluded because the SEMS indication was anastomotic fistula or post-esophagectomy stenosis. Of the remaining 335 patients, 37 (11.0%) developed a SEMS-ERF. The median time to ERF development was 129 days (range, 35–646 days) (Fig. 2).
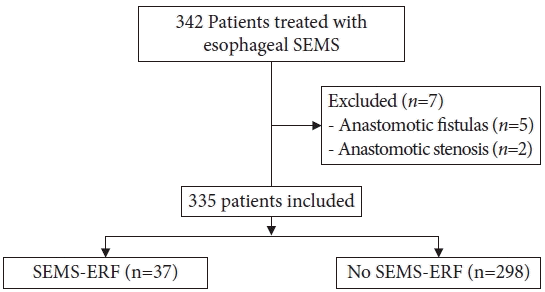
Flowchart of patients included in the study. SEMS, self-expandable metallic stent; SEMS-ERF, SEMS-induced esophagorespiratory fistula.
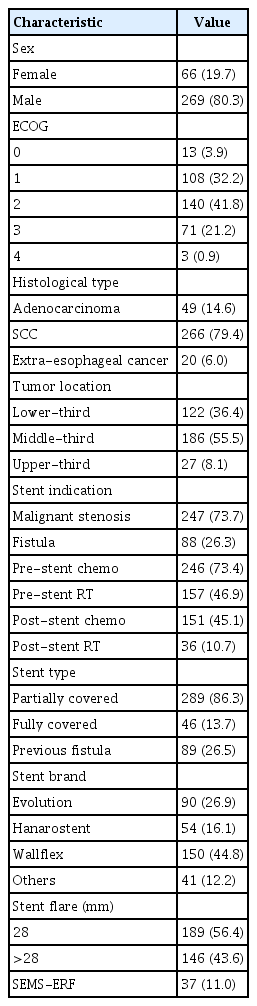
Table 1 summarizes the patients’ characteristics and SEMS-ERF incidence. The majority of the patients were male (80.3%), with a mean age of 61.6 years (range, 21–94 years). Most patients had squamous cell carcinoma (SCC) (79.4%), and 55.5% of all the tumors were located in the middle third of the esophagus. Forty-nine patients (14.6%) had adenocarcinoma; 91% were located in the distal esophagus, and 9% were located in the middle esophagus. Seventy-three percent of the patients received chemotherapy before stent placement, and 46.9% had prior RT. Partially covered stents were used in 86% of the patients, and 56.4% of the stents had a flare diameter <28 mm.
Risk factors associated with SEMS-ERF
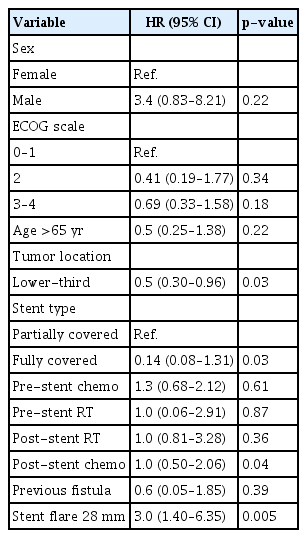
In the Cox HR analysis, only stent flare of 28 mm (HR, 3.05; 95% CI, 1.40–6.35; p=0.005) was a risk factor for SEMS-ERF. Tumors located in the lower third portion of the esophagus were protective factors (HR, 0.5; 95% CI, 0.30–0.96; p=0.03). Table 2 summarizes the analyzed variables.
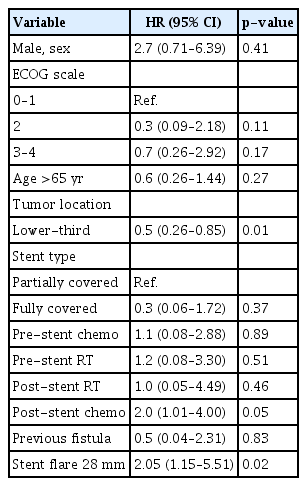
Using mortality as a competing risk factor, the analysis indicated a stent flare of 28 mm (HR, 2.05; 95% CI, 1.15–5.51; p=0.02) and post-stent chemotherapy (HR, 2.0; 95% CI, 1.01–4.00; p=0.05) as risk factors for SEMS-ERF. Lower-third portion tumors were identified as a protective factor (HR, 0.5; 95% CI, 0.26–0.85; p=0.01). Table 3 summarizes the analysis.
Risk factors for SEMS-ERF in patients with SCC
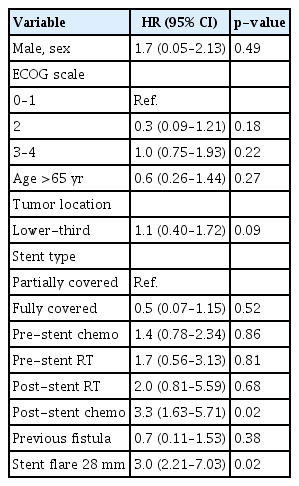
Since no patient with adenocarcinoma developed SEMS-ERF, a secondary analysis was performed, excluding these patients. In the Cox HR analysis, only stent flare of 28 mm (HR, 3.0; 95% CI, 1.40–6.35; p=0.005) was a risk factor for SEMS-ERF, while lower-third tumors were a protective factor (HR, 0.5; 95% CI, 0.30–0.96, p=0.03). Table 4 summarizes the analysis.

Risk factors for the occurrence of fistula in patients with squamous cell carcinoma: Cox HR analysis
Using mortality as a competing risk factor, the analysis identified a stent flare of 28 mm (HR, 3.0; 95% CI, 2.21–7.03; p=0.02) and post-stent chemotherapy (HR, 3.3; 95% CI, 1.63–5.71; p=0.02) as risk factors for SEMS-ERF. Table 5 summarizes the analyzed variables.
Management of SEMS-ERF
Regarding the approach of patients diagnosed with SEMS-ERF (n=37), 14 patients received a second SEMS placed over the fistula to provide a seal. Successful fistula sealing was achieved in all patients, and all patients were able to resume oral feeding. The remaining 23 patients were considered too debilitated to resume oral feeding even if the fistula was successfully sealed with a second stent. Nineteen of these patients were treated with feeding tube placement, three underwent gastrostomy, and one underwent a jejunostomy.
Survival
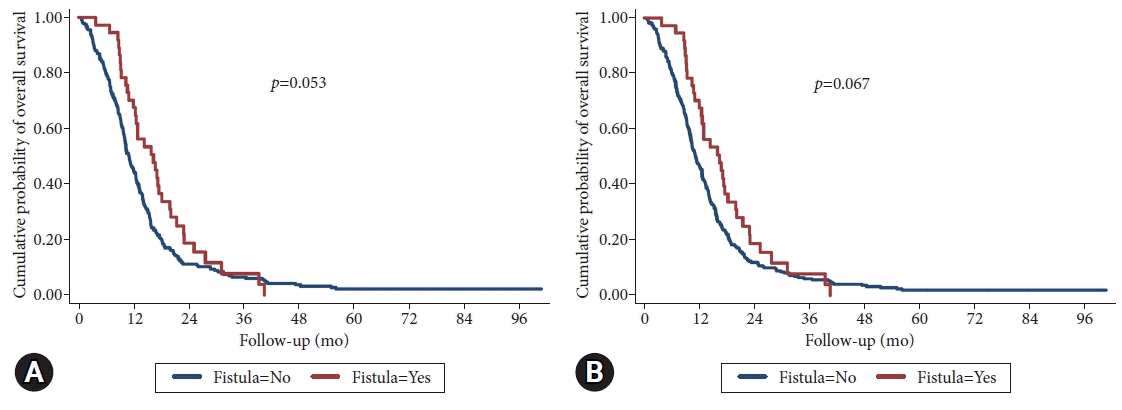
The median overall survival was 11.8 months, ranging from 0.4–100 months). In patients with SEMS-ERF, the median survival was 16.3 months, while in patients without SEMS-ERF, it was 11.2 months (log-rank test, p=0.053; Fig. 3). When patients with adenocarcinoma were excluded from the group without SEMS-ERF, the median survivals was 16.3 months and 11 months in SEMS-ERF and without SEMS-ERF, respectively (log-rank test, p=0.067; Fig. 3). The median survival after the development of SEMS-ERF was 3.6 months, ranging from 1 to 455 days.
DISCUSSION
Currently, SEMS placement is the most accepted and widely adopted method for the palliation of dysphagia in patients with esophageal cancer.7-9 It is also the preferred intervention for malignant esophageal fistulas.10,11 As already reported in previous studies,2,4,12,13 SEMS-ERF is an expected AE in the palliative treatment of dysphagia. We could not determine the significant impact of the SEMS-ERF on mortality. However, 62.2% (23/37) of patients diagnosed with SEMS-ERF were not clinically eligible for a second stent, suggesting an increase in morbidity caused by the fistula. Despite its importance, little is known about what leads to fistula development. This is the first study to assess the risk factors for SEMS-ERF, specifically in patients with malignant disease.
In our study, the incidence of SEMS-ERFs was 11.0% (37/335). Homann et al.4 analyzed delayed (>4 weeks) AEs of stent placement in 133 patients with esophageal cancer and reported SEMS-ERFs in 9% of them. A retrospective study including patients with benign and malignant indications for SEMS reported an overall incidence of 4%.6 However, only 9% of the patients had the malignant disease. Esophageal (upper-third portion) cancer has been identified as a risk factor for SEMS-ERF development. In our study, we could not corroborate these data; however, lower-third portion tumors were found to be protective factors.
The stent diameter was identified as a risk factor for the development of SEMS-ERFs. Although another study6 analyzed stent size in patients with ERF, they were not able to find a significant difference, possibly because of the small sample size (n=21). In our analysis, a stent flare diameter of 28 mm (HR, 2.05; 95% CI, 1.15–5.51; p=0.02) showed a higher risk of ERF development. In a small series, Bethge et al.14 analyzed microscopical samples and described necrosis and ischemia at the sites of contact between the stent and esophageal mucosa. In our opinion, this suggests that the development of SEMS-ERF is more related to stent-induced ischemia rather than the presence of infiltrative neoplasia.
The histological type has not been highlighted as an important factor in SEMS-ERF development in the literature. Some studies4-6 reported data on the histological type, but there was no comparison between groups. In our study, we found no cases of SEMS-ERF in patients with adenocarcinoma (0/49), whereas 35 of the 37 SEMS-ERF cases were reported in patients with SCC. In our opinion, this finding can be explained by tumor location, as adenocarcinoma is more common in the lower-third esophagus, away from the airway, and therefore, less likely to develop SEMS-ERF. We also performed a separate analysis of patients with SCC; however, the results were similar to those of the overall analysis.
The impact of chemotherapy on the development of SEMS-related AEs remains controversial. Medeiros et al.3 found a trend toward higher AE rates in patients who received adjuvant therapy. Other studies6,7 found an association between prior chemotherapy and stent-related AEs, especially migration. In our study, we found an association between post-stent placement chemotherapy and the development of SEMS-ERF (HR, 2.0; 95% CI, 1.01–4.00; p=0.05). This relationship was not observed in patients who received combined therapy (RT+chemotherapy). In our opinion, patients with a better performance status are more likely to receive systemic therapy. With longer survival, these patients are more likely to live long enough to develop ERF, as the median time for ERF development was 129 days. However, we could not find data statistically supporting the relationship between longer survival and SEMS-ERF development.
The effect of RT on the development of stent-related AE is a matter of intense debate. Many studies15-19 reported a higher risk of overall AEs in patients who underwent RT after stent placement. A recent guideline20 advises against stent placement concomitant with RT. However, our experience21 is in agreement with other studies22 that reported that only minor AEs (mainly mild chest pain) occurred more frequently in patients receiving RT. Regarding the risk of ERF, Bick et al.6 showed a 9.4-fold greater chance of ERF development in patients with a history of RT. In our study, we found no relationship between RT and a higher risk of ERF, either alone or in combination with chemotherapy.
Our study had some limitations, as it was a single-center retrospective study. Quality-of-life questionnaires were not applied, limiting the assessment of SEMS-ERF morbidity. Over the years, many types, sizes, and brands of stents have been used, implicating heterogeneity and possible biases. In addition, it was not possible to include disease progression as a risk factor for SEMS-ERF because all patients included in this study already had advanced terminal cancer. However, we managed to present a study with the largest sample of SEMS-ERFs to date, adding information to the limited literature on this subject.
In conclusion, the incidence of SEMS-ERFs in our study was 11%, with a median time of 129 days after SEMS placement. Post-stent chemotherapy and a 28 mm stent flare were associated with a higher risk of SEMS-ERF. For patients with these risk factors undergoing stent placement, stents with smaller flare diameters are recommended. Clinicians should maintain a high level of suspicion for SEMS-ERF since early intervention with additional stent placement may reduce morbidity.
Notes
Conflicts of Interest
The authors have no potential conflicts of interest.
Funding
None.
Acknowledgments
The authors would like to thank all nurses, doctors, and other employees of the GI Endoscopy Department at the Instituto do Cancer do Estado de São Paulo. It requires great individual commitment to achieve great teamwork. We also appreciate all other professionals involved in the care of patients included in this study for their indirect contribution.
Author Contributions
Conceptualization: IRJ, BCM, AAM, FMF; Data curation: IRJ, BCM, AAM, GRAL, MACC, AAMP; Formal analysis: IRJ, BCM, AAM, GRAL, MACC, AAMP; Supervision: RAAS, UR, THB, FMF; Validation: RAAS, UR, THB, FMF; Writing–original draft: IRJ, BM, FMF; Writing–review & editing: all authors.