22-gauge Co-Cr versus stainless-steel Franseen needles for endoscopic ultrasound-guided tissue acquisition in patients with solid pancreatic lesions
Article information
Abstract
Background/Aims
Endoscopic ultrasound-guided tissue acquisition (EUS-TA) using Franseen needles is reportedly useful for its high diagnostic yield. This study compared the diagnostic yield and puncturing ability of EUS-TA using 22-gauge cobalt-chromium (CO-Cr) needles with those of stainless-steel Franseen needles in patients with solid pancreatic lesions.
Methods
Outcomes were compared between the 22-gauge Co-Cr Franseen needle (December 2019 to November 2020; group C) and stainless-steel needle (November 2020 to May 2022; group S).
Results
A total of 155 patients (group C, 75; group S, 80) were eligible. The diagnostic accuracy was 92.0% in group C and 96.3% in group S with no significant intergroup differences (p=0.32). The rate of change in the operator (from training fellows to experts) was 20.0% (15/75) in group C and 7.5% (6/80) in group S. Stainless-steel Franseen needles showed less inter-operator difference than Co-Cr needles (p=0.03).
Conclusions
Both Co-Cr and stainless-steel Franseen needles showed high diagnostic ability. Stainless-steel Franseen needles are soft and flexible; therefore, the range of puncture angles can be widely adjusted, making them suitable for training fellows to complete the procedure.
INTRODUCTION
Although imaging modalities such as computed tomography (CT) and magnetic resonance imaging (MRI) are useful for detecting solid pancreatic lesions, distinguishing between benign and malignant lesions is sometimes difficult. Endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA) was introduced in 1992; since then, EUS-guided tissue acquisition (EUS-TA) became an established procedure for acquiring tissue samples to make a histological diagnosis of solid pancreatic lesions.1-3 However, it can be difficult to obtain adequate specimens using EUS-FNA needles when immunostaining is required, such as in patients with neuroendocrine neoplasm (NEN). Although EUS-TA using a 19-gauge FNA needles is considered useful in such cases since it is expected to obtain sufficient specimens for immunostaining, its thick needle is thought to have high puncture resistance, making the procedure difficult.4 To address these limitations, fine-needle biopsy (FNB) needles, which are primarily used to obtain core tissue samples, are reportedly useful.5-8 In a recent multicenter randomized control study, the diagnostic accuracy and adverse event rates of EUS-TA using FNB needles were 94.7% and 0.7%, respectively,9 proving its efficacy and safety. Moreover, EUS-TA using FNB needles plays a crucial role in obtaining sufficient tissues to enable personalized medicine involving next-generation sequencing (NGS) in patients with pancreatic cancer.10,11
Among FNB needle types, the 22-gauge cobalt-chromium (CO-Cr) Franseen needle has been widely used for tissue acquisition with a high diagnostic yield.12-15 As Co-Cr materials are harder than stainless-steel needles, they are considered durable for repeated punctures without needle dysfunction. However, it may be difficult to adjust the appropriate EUS viewing to achieve puncture because of its stiffness and limited range of puncture angles compared to stainless-steel needles. Therefore, a stainless-steel Franseen needle was recently designed, and the limitations of the Co-Cr Franseen needle require addressing because of the increased flexibility of stainless steel. As EUS-TA using FNB needles is increasingly used, more endoscopists are performing this procedure; therefore, the development of FNB needles that puncture easily, even for training fellows, is warranted. It would be worthwhile to evaluate the diagnostic yield and puncture ability of EUS-TA using a stainless-steel Franseen needle. Hence, here we compared the diagnostic yield and puncturing ability of 22-gauge Co-Cr needles with those of stainless-steel Franseen needles in patients with solid pancreatic lesions.
METHODS
Patients and Franseen needles
The data of patients with solid pancreatic lesions who underwent EUS-TA using Franseen needles between December 2019 and May 2022 were retrospectively evaluated at our facility. The inclusion criteria were as follows: solid pancreatic lesions detected by imaging modalities such as CT or MRI; and required histological diagnosis for surgery, chemotherapy, or observation. The exclusion criteria were as follows: treatment with anti‑thrombotic agents that could not be discontinued; and switch from 22-gauge Franseen needles to 25-gauge needles due to puncture difficulty.
The patients were divided into those who underwent EUS-TA using 22-gauge Co-Cr Franseen needles (group C: Acquire; Boston Scientific Corp., December 2019 to November 2020); and those who underwent EUS-TA using a 22-gauge stainless-steel Franseen needle (group S: SonoTip TopGain; Medi-Globe GmbH, December 2020 to May 2022) (Fig. 1). Both needles have three symmetrical heels designed to maximize tissue capture and minimize fragmentation. No intergroup crossover was observed.
Procedures
EUS-TA using Franseen needles was performed by six training fellows with experience performing more than 1,000 esophagogastroduodenoscopies, 500 colonoscopies, and 20 EUS procedures (observation); however, fewer than 30 EUS-TA procedures were independently included during the study period. The trainees performed EUS-TA with expert endoscopists who had performed 100 EUS-TA procedures. If it was difficult to train the fellows, the expert endoscopists performed the procedure instead. Three training fellows performed the procedures in each group with no crossover between them. Patients were under conscious sedation with midazolam and pethidine.
EUS-TA was performed using a linear EUS (GF‑UCT260; Olympus Marketing). After the solid pancreatic lesion was visualized and the surrounding vasculature was assessed, the lesion was punctured through the stomach or duodenum. The stylet was withdrawn and negative suction was applied using a 20-mL vacuum syringe. If blood was visible in the syringe during the first puncture, a slow-pull technique16-18 or no suction was applied during the second puncture to avoid blood contamination. Approximately 10 to 20 rapid strokes were performed within the lesion, followed by suction release and needle removal. Tissue specimens were then pushed onto glass slides using a stylet or air pressure.
Histologic evaluation
As rapid on-site evaluation19,20 was unavailable, we repeatedly punctured the area under discussion with a cytological technician until sufficient samples were obtained for histopathological diagnosis or immunostaining. The color of the specimens was then checked, with red specimens considered blood elements and white specimens considered tissues. All samples obtained by EUS-TA were mixed and placed in a container containing 10% neutral-buffered formalin. The samples were then assessed for adequacy and preserved in formalin, embedded in paraffin, and cut into 4-µm-thick serial sections for hematoxylin and eosin staining for histological diagnosis, followed by immunostaining if needed. Two pathologists who were blinded to the needles used performed the histological analyses.
Definitions and outcome measurement
The following information was extracted from patient medical records: age, sex, lesion size, lesion location, puncture site, treatment method (surgery, chemotherapy, or observation), and final diagnosis. We made the final histological diagnosis using surgical specimens from patients who had undergone surgery. For patients treated non-surgically, we confirmed the final malignant diagnosis histologically using the EUS‑TA-based tissue and when the clinical course and follow-up imaging evaluations worsened. In cases that could not be diagnosed histologically, a final diagnosis of malignancy was made when the clinical course or imaging findings worsened. Benign lesions (e.g., focal pancreatitis) were diagnosed if EUS-TA showed no malignant findings and imaging modalities showed no exacerbation after 6 months of follow-up.
To compare the procedural outcomes and diagnostic yields of Co-Cr (group C) and stainless-steel Franseen needles (group S), the procedure outcomes included the number of passes, procedure time, technical success, change in the operator, and adverse event rates. The number of passes was defined as the number of punctures required to complete EUS-TA. The procedure time was defined as the duration from its beginning to its end. Technical success was defined as the successful acquisition of macroscopically visible whitish material by EUS-TA. A change in the operator was defined as the operator changing from a training fellow to an expert during the EUS-TA procedure (e.g., when it was impossible to penetrate the lesion using Franseen needles or impossible to visualize the lesion at the appropriate location for puncture). Adverse event severity was graded according to the American Society for Gastrointestinal Endoscopy lexicon.21
Statistical analysis
All findings, including patient characteristics, outcomes, and diagnostic yields, were compared between the Co-Cr (group C) and stainless-steel (group S) Franseen needles. The diagnostic yields included the sensitivity, specificity, positive predictive value, negative predictive value, and accuracy of EUS-TA using the Co-Cr and stainless-steel Franseen needles. Binary variables were compared using Fisher’s exact test, while continuous variables were compared using the Mann–Whitney U test. Differences with p<0.05 were considered significant. Statistical analyses were performed using STATA ver. 17 software (StataCorp.).
Ethical statements
This retrospective study was approved by the Institutional Review Board of Saitama Medical University International Medical Center (approval number: 18-253). All patients who underwent EUS-TA provided written informed consent before the procedure; however, the need for informed consent was waived via an opt-out form.
RESULTS
Patients and training fellows
Figure 2 shows a flowchart of patient eligibility for this study. In the Co-Cr group (December 2019 to November 2020), 80 patients were initially included. We excluded one patient who received antithrombotic therapy and four who were treated with a 25-gauge FNA needle. Finally, 75 patients were included in the Co-Cr Franseen needle group (group C).

Patient eligibility flowchart. EUS-TA, endoscopic ultrasound-guided tissue acquisition; FNA, fine-needle aspiration.
In the stainless-steel group (December 2020 to May 2022), 83 patients were initially included. After we excluded two patients who received antithrombotic therapy and one who was punctured with a 25-gauge FNA needle, 80 patients were ultimately eligible for inclusion in the stainless-steel Franseen needle group (group S). A total of 155 patients (group C, 75; group S, 80) were evaluated. The reason for using a 25-gauge FNA needle in both groups was the difficulty of puncturing with 22-gauge Franseen needles because of the tiny (<15 mm) lesions positioned near the vessel that could not be avoided.
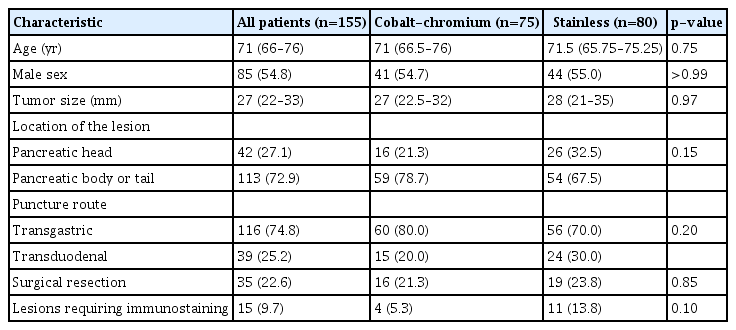
The patient characteristics of groups C and S are shown in Table 1. Age, sex, lesion size, lesion location, puncture route, surgical resection, and immunostaining requirement did not differ significantly between groups.
The experiences of the training fellows who underwent EUS-TA are shown in Table 2. The experience with esophagogastroduodenoscopy, EUS (observation), and prior EUS-TA between groups C (trainees A–C) and S (trainees D–F) were similar.
Final diagnosis
Table 3 presents the final diagnoses of the solid pancreatic lesions. Adenocarcinoma was the most common (n=128; group C, 60; group S, 68), followed by benign lesions (n=19; group C, 11; group S, 8) and NEN (n=8; group C, 4; group S, 4). There were no significant intergroup differences.
Procedure outcome and diagnostic yields of EUS-TA
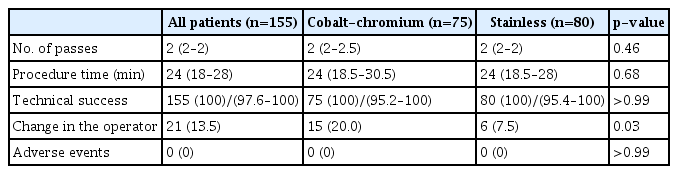
Table 4 compares the procedural outcomes between the Co-Cr (group C) and stainless-steel (group S) Franseen needle groups. The operator change rate was lower in group S (7.5%, 6/80) than in group C (20.0%, 15/75; p=0.03). The reasons for changing the operator in each group were as follows: in group C, there were seven cases in which it was difficult to adjust the puncture route from the second portion of the duodenum, five cases in which it was difficult to penetrate the gastric wall, and three cases in which it was difficult to penetrate the lesions (transgastric route). In contrast, in group S, difficulty was encountered in adjusting the puncture route from the second portion of the duodenum in one patient, difficulty was encountered in penetrating the gastric wall, and difficulty was encountered in avoiding the vessels. While all operator changes in group C occurred during the first puncture, one case of difficulty penetrating the gastric wall occurred during the third puncture and two cases with difficulty avoiding vessels occurred during the second and third punctures in group S. All cases were successfully punctured after an expert took over. The number of passes, procedure time, technical success rate, and adverse event rate did not differ significantly between groups.
Table 5 shows the diagnostic yields of EUS-TA by study group. The diagnostic accuracies of EUS-TA in groups C and S in all cases were 92.0% (95% confidence interval [CI], 83.3%–96.6%) and 96.3% (95% CI, 89.1%–99.2%), respectively, with no significant difference (p=0.32). Other outcomes for the diagnosis of malignancy, such as sensitivity and specificity, were also not significantly different between groups, nor were the diagnostic accuracies of transgastric puncture, transduodenal puncture, and lesions within 20 mm.
DISCUSSION
Considering the recent widespread adoption of EUS-TA using FNB needles, thorough knowledge of the characteristics of FNB needle types is essential to their appropriate selection by lesion or endoscopist characteristics. Therefore, the present study compared Franseen needles of different materials. The present study compared 75 cases treated with Co-Cr (group C) and 80 cases treated with stainless-steel Franseen needles (group S). Patient characteristics did not differ significantly between groups. Therefore, we directly compared the procedural outcomes and diagnostic yields of EUS-TA.
Considering procedural outcomes, stainless-steel Franseen needles showed less change in the operator from training fellows to experts than Co-Cr needles. Noteworthy, the most common reason for changing the operator was difficulty adjusting the puncture route from the second portion of the duodenum, occurring in 9.3% (7/75) of group C versus 1.3% (1/80) of group S cases, showing a significant intergroup difference (p=0.03). When the elevator of the linear echoendoscope was fully used, the Co-Cr needles provided a puncture angle similar to that of the stainless-steel needles when EUS-TA was performed from the stomach (Fig. 3A, B). Puncture from the duodenum (in particular, the second portion of the duodenum) is considered difficult regardless of needle type, as the lesion is usually visualized while withdrawing the scope at a full angle. Moreover, needle flexibility decreases in situations in which the scope is subjected to strong stress. As the Co-Cr needle is harder than the stainless-steel needle, the difference in flexibility between them for duodenal puncture seems more significant than that in punctures of other parts (Fig. 3C, D). Therefore, the trainees using the Co-Cr needles seemed to have had difficulty puncturing lesions from the second part of the duodenum, as they could not achieve an appropriate EUS view to make the puncture because of the limited puncture angle range. Although experts successfully performed EUS-TA using Co-Cr needles from the second part of the duodenum in all cases, they also experienced puncture difficulty. However, they managed to fix the lesion by achieving appropriate tension (while withdrawing) of the scope at a full angle, which provided better EUS viewing to achieve the puncture. Conversely, the stainless-steel needle is flexible, allowing a wider range of puncture angles; therefore, it is easier to train fellows to puncture using stainless-steel than Co-Cr needles. Increasing numbers of endoscopists are performing EUS-TA. Therefore, education regarding it is very important, and flexible FNB needles may be a good option for completing this procedure. We believe that this is new evidence.

Endoscopic ultrasound findings. Endoscopic ultrasound-guided tissue acquisition (EUS-TA) using cobalt-chromium (CO-Cr) (A) and stainless-steel Franseen needle (B) from the stomach when the elevator of the linear echoendoscope was fully used. Both needles are visible (pink arrows). No significant difference intergroup difference in puncture angles was noted. EUS-TA using Co-Cr (C) and stainless-steel Franseen needles (D) from the second portion of the duodenum when the elevator of the linear echoendoscope was fully used. Both needles are visible (pink arrows). The stainless-steel needles had a greater puncture angle than the Co-Cr needles.
Among the cases of operator turnover due to unsuccessful penetration of the gastric wall and lesions (8/75) in group C, three were due to difficulty penetrating the lesions via the transgastric route. As all of these lesions were ≤20 mm in diameter, it seemed difficult for trainee fellows to penetrate them using 22-gauge needles compared to larger lesions regardless of needle type. Moreover, operator turnover due to unsuccessful penetration of the gastric wall between groups C and S was 6.7% (5/75) and 2.5% (2/80), respectively. The differences between the groups were not statistically significant (p=0.26). Therefore, we believe that the penetration performance of the two groups in our study was similar. In cases of unsuccessful penetration of the gastric wall and lesions by trainee fellows, experts successfully penetrated them using an appropriate upward angle and holding the gastric wall and lesions at the tip of the endoscope. This technique fixes the gastric wall and lesions to facilitate penetration.
Other than the change in the operator, no significant intergroup differences were observed. In most cases, the procedure was completed by a second puncture in both groups. This could have been a result of the larger tissues that were collected with the Franseen needle, which is an advantage of FNB needles. The Franseen needle tip, which has three symmetric heels, may cause trauma and adverse events such as bleeding and pancreatitis. No adverse events were observed in this study, and the minimal puncturing may have prevented these adverse events. However, we must consider that adverse events can occur with Franseen needle use.
Both study groups showed acceptable diagnostic accuracy compared to a recent multicenter randomized controlled study. Although transduodenal puncture using Franseen needles is reportedly difficult,15 both groups showed acceptable diagnostic accuracy in this study.
Although we demonstrated the advantage of stainless-steel over Co-Cr Franseen needles in this study, stainless-steel needles also have the disadvantage of causing needle dysfunction after many punctures owing to their softness. This causes the tip of the needle to bend and complicates visualizing the needle tip in EUS viewing, which in turn increases the risk of adverse events such as bleeding and pancreatitis. In fact, among the cases in which the operator was changed in group S, one case of difficulty penetrating the gastric wall occurred during the third puncture. This might be due to needle dysfunction, which results in difficulty penetrating the gastric wall. Moreover, there were two cases of difficulty avoiding vessels during the second and third punctures. This was due to the poorer visibility of the needle than that in the first puncture caused by needle dysfunction, which led the needle tip to bend. These outcomes demonstrate needle dysfunction concerns with stainless-steel Franseen needles. Moreover, in another study, two of 50 cases treated with stainless-steel needles experienced needle dysfunction after the first puncture.22 In contrast, the cases of operator change in group C all occurred during the first puncture. This demonstrates that the cause of operator change with Co-Cr Franseen needle use was not needle dysfunction. Therefore, when multiple punctures are expected (e.g., when NGS tissues are required), it may be better to select a Co-Cr needle. This emphasizes the importance of selecting an appropriate needle to achieve an effective and safe EUS-TA procedure depending on the indication.
Our study had some limitations. First, this was a single-center retrospective study and not a randomized controlled study. Second, two different types of needles were used at different time points, and EUS-TA was performed by different endoscopists in both groups. Third, the heterogeneity of training fellows may have affected the rates of change in the operator, although the three training fellows had similar experience and did not cross groups. Moreover, this may have affected other outcomes despite the training fellows performing all EUS-TA procedures with experts to ensure their safety and efficacy. Further studies evaluating EUS-TA using both types of needle during the same period performed by the same training fellow are warranted.
In conclusion, both Co-Cr and stainless-steel Franseen needles used for EUS-TA showed high diagnostic ability for pancreatic solid lesions in this study. Because stainless-steel Franseen needles are soft and flexible, the range of the puncture angle can be widely adjusted, suggesting their better suitability for training fellows completing this procedure. When selecting needles for EUS-TA, it is important to consider that the appropriate needle differs depending on the lesion, endoscopist, and purpose.
Notes
Conflicts of Interest
The authors have no potential conflicts of interest.
Funding
None.
Acknowledgments
We are indebted to Prof. Masanori Yasuda for diagnosing the pathological findings.
Author Contributions
Conceptualization: YT; Data curation: YT, AF; Formal analysis: YT, RJ; Investigation: YT, MM, AF, RS, TS, KS, TT, YM, SR; Methodology: YT, SR; Project administration: YT, SR; Supervision: MM, SR; Validation: RJ; Writing–original draft: YT; Writing–review & editing: all authors.