Role of Advanced Endoscopic Imaging Techniques in the Management of Inflammatory Bowel Disease
Article information
Abstract
Endoscopy plays a crucial role in the management of inflammatory bowel disease (IBD) in terms of diagnosis, monitoring of mucosal status, and surveillance of colitis-associated neoplasia. Mucosal healing evaluated by endoscopy has been recognized as the target of treatment in the era of powerful biologics therapy. The optimal modality for identifying dysplasia in IBD has yet to be well defined. Increasing progress has recently been made in endoscopic technologies to more accurately assess mucosal inflammation and more effectively detect dysplasia. Here we review the data of advanced endoscopic imaging techniques such as chromoendoscopy, virtual chromoendoscopy, endocytoscopy, and confocal laser endomicroscopy in the management of IBD.
INTRODUCTION
Inflammatory bowel disease (IBD), consisting of Crohn’s disease (CD) and ulcerative colitis (UC), is characterized by chronic inflammation in the digestive tract and often requires lifelong management. The diagnosis of IBD is challenging, as there is no single gold standard diagnostic tool. It is usually established based on various evaluating modalities like clinical assessment, laboratory tests, endoscopy, radiologic evaluations, and histology [1-3]. Among them, endoscopy is of paramount importance for making the correct diagnosis. In addition, endoscopy is crucial for the assessment of disease activity, which is required for proper therapeutic planning. Powerful biologic therapy has brought a paradigm shift in the therapeutic target from clinical symptoms to mucosal healing that can be observed through endoscopy. Growing evidence shows that mucosal healing is associated with many favorable outcomes such as lower hospitalization rates, reduced disease relapse rates, and lower surgical rates [4,5]. Therefore, the role of endoscopy in IBD is more essential now than before.
Patients with long-standing IBD are at an increased risk of colitis-associated colorectal cancer (CRC) [6-8]. Studies with long-term follow-up periods have shown that the 10-, 20-, and 30-year incidences of CRC in patients with UC are 2%, 8%, and 18%, respectively, which are significantly higher than those in the general population [9]. Thus, patients with IBD should undergo regular endoscopy to enable the early detection of CRC and precancerous lesion like dysplasia, leading to better prognosis. However, colitis-associated neoplastic lesions in patients with IBD are extremely difficult to find because they tend to be flat and indistinguishable against the background mucosa due to the surrounding chronic inflammation [10,11].
Recently, rapid advancements in endoscopic technologies including chromoendoscopy (CE), virtual CE, endocytoscopy, and confocal laser endomicroscopy (CLE) have enabled more accurate assessment of mucosal inflammation and more effective unveiling of dysplasia. In this review, we will discuss the role of these advanced endoscopic imaging techniques in the management in IBD and provide updated data in this field.
CHROMOENDOSCOPY
Dye CE, which was introduced more than a decade ago, uses dye agents to enhance the detection of mucosal lesions. These agents include absorptive agents (methylene blue, toluidine blue, and cresyl violet), contrast agents (indigo carmine and acetic acid), and reactive staining agents (Congo red and phenol red) [12]. Among them, methylene blue and indigo carmine are the main agents for dye CE in IBD patients.
Dye CE shows a clear benefit for the detection of neoplasia in the context of IBD surveillance. Significantly more intraepithelial neoplastic lesions were found in the indigo carmine–aided CE group than in the control group using magnification with conventional white-light endoscopy in 350 patients with long-standing UC (69 vs. 24, p<0.0001) [13]. Methylene blue–assisted CE also reports a notable 3.2-fold increase in the dysplasia detection rate in UC compared with white-light endoscopy [14]. In a prospective study, six endoscopists conducted white-light endoscopy followed by indigo carmine–based CE in 75 patients with UC [15]. It showed a significantly better dysplasia detection rate (21.3% vs. 9.3%, p=0.007) and similar high rate of interobserver agreement for polyp detection (kappa score 0.86 vs. 0.91) as compared with white-light endoscopy. Enhanced dysplasia detection of dye CE in UC was confirmed in a meta-analysis of six randomized controlled trials demonstrating a pooled sensitivity of 83%, specificity of 91%, and diagnostic odds ratio of 17.5 [16]. According to these results, dye CE is currently recommended by most guidelines as an alternative to non-targeted random biopsies for dysplasia surveillance in long-standing UC [17,18]. Dye CE also provides an accurate diagnosis of inflammation activity extent and severity in patients with UC [19,20].
Despite the above advantages, dye CE is not accepted as an optimal technique in routine clinical practice due to the following limitations [21]. First, it takes more time (dye CE increases procedure time by around 10 min). Second, it requires operator training and the additional cost of the dye. Finally, there has yet to be a study demonstrating a significant advantage of using dye CE in terms of CRC-related morbidity and mortality.
Recent advances in endoscopic imaging technology allow the use of CE without spraying dye agents during colonoscopy. This dye-less CE or image enhanced endoscopy can be performed by just a click of a button with no need to apply specialized equipment in the middle of the procedure. Choices include narrow band imaging (NBI; Olympus, Tokyo, Japan), Fuji Intelligent Color Enhancement (FICE; Fujinon, Tokyo, Japan), and the i-scan (Pentax, Tokyo, Japan). NBI uses an optic filter that narrows down the spectrum of light emitted from the scope, resulting in better visualization of the mucosal vascularity [22]. FICE and the i-scan enhance images in different ways but use the same physical principle as NBI, which emphasizes the intensity of blue light. Instead of using optical filters inside of the endoscope, they digitally reconstruct virtual images in real time, using computed spectral estimation technology [23]. Most studies were conducted using NBI.
Several studies have evaluated the role of NBI for predicting and detecting neoplasia in UC. A pilot study using magnifying endoscopy with NBI in 46 patients with UC showed the potential benefit of NBI to predict dysplasia [24]. They analyzed the surface pattern and classified it into honeycomb (n=161), villous (n=85), and tortuous (n=50). The positive rate of dysplasia was higher in the tortuous pattern than in other patterns (8% vs. 0.4%, p=0.003) suggesting that the former recognized by NBI may predict the presence of dysplasia in UC. However, prospective studies failed to reveal a significant advantage of NBI in terms of surveillance of dysplasia in UC compared with white-light endoscopy. A randomized crossover study comparing white-light endoscopy with NBI in 48 patients with extensive UC for >8 years showed no significant difference in the detection of dysplasia between groups (NBI, 13 lesions vs. white-light endoscopy, 11 lesions, p=0.727) [25]. In another randomized study of 112 patients with long-standing UC, there was no difference in dysplasia detection rates of 9% in both arms [26]. A recent randomized study including a large number of UC patients (n=159) also showed a comparable number of neoplasia with both techniques, indicating that NBI does not improve the detection of neoplasia in UC compared with white-light endoscopy [27]. The only benefits of NBI over white-light endoscopy are fewer biopsy specimens and less withdrawal time. However, these prospective NBI studies did not use magnifying endoscopy. High magnification in combination with dye CE seemed to show a clear benefit over whitelight colonoscopy for detecting neoplastic lesions in UC [13,28]. Therefore, a prospective study to evaluate the efficacy of magnifying NBI for colitis-associated dysplasia in UC is needed.
Several studies have determined the effect of dye-less CE compared with dye CE for the detection of neoplasia in IBD [29-31]. In a nutshell, NBI is not superior to dye CE for this purpose. A prospective crossover study comparing indigo carmine–based CE with NBI for the detection of dysplasia in 60 IBD patients showed a higher neoplasia miss rate in the NBI group than in the dye CE group [31]. Therefore, NBI cannot be recommended as the standard technique for surveillance in IBD. There is a lack of studies using FICE or the i-scan for detecting neoplasia in IBD patients.
Studies have shown a vital role of dye-less CE in the assessment of mucosal inflammation. In a study using magnifying NBI in 60 patients with IBD (17 CD and 43 UC) and 24 control participants, patients with CD and UC had branchlike structures more frequently and higher vascularity of the domes in Peyer’s patches than control subjects [32]. A randomized study comparing high-definition white-light endoscopy and the i-scan for detecting mucosal inflammation in 78 patients with IBD reported that the i-scan showed better agreement than white-light endoscopy with the histologic findings in extent (92.3% vs. 48.7%) and degree (89.7% vs. 53.9%) of inflammatory activity [33]. Another study evaluated the value of NBI for assessing specific mucosal vascular patterns found in 67% of colorectal segments from 30 UC patients [34]. Mucosal inflammation indicators such as acute inflammatory cell infiltrates, goblet cell depletion, and basal plasmacytosis were significantly more noted in patients with altered mucosal vascular pattern than in those with a normal pattern. Since mucosal healing is recognized as an important therapeutic endpoint in IBD, efforts have been made to evaluate mucosal healing status more accurately with enhanced endoscopic techniques. Very recently, a new endoscopic score for assessing the severity of mucosal inflammation in UC using the i-scan has been developed and validated, showing very good interobserver agreement [35]. It defined and characterized the endoscopic mucosal and vascular healing, reflecting the full range of histologic changes. Taken together, these results indicate that dye-less CE can offer a precise assessment of mucosal inflammatory extent and severity of IBD patients.
ENDOCYTOSCOPY
Endocytoscopy is a novel technique enabling the real-time microscopic imaging of gastrointestinal mucosa with a magnification power up to 1400-fold [36]. It is based on contact light microscopy along with preparation of the mucosal layer using absorptive contrast agents such as methylene blue or toluidine blue. In a pilot study of 40 IBD patients, endocytoscopy reliably differentiated single inflammatory cells with good sensitivity and specificity (neutrophils, 60% and 95%; basophils, 74.4% and 94.4%; eosinophilic granulocytes, 75% and 90.5%; and lymphocytes, 88.9% and 93.3%) [37]. Furthermore, the agreement rate between endocytoscopy and histology for grading intestinal inflammation was 100%. However, the real clinical implication of this technique in IBD remains to be seen because data regarding this issue are still lacking. Further, there are no data on the role of endocytoscopy in the surveillance of colitis-associated neoplasia.
CONFOCAL LASER ENDOMICROSCOPY
CLE is also able to offer real-time in vivo histology of the intestinal mucosa with 1000-fold magnification power during endoscopy [38]. It requires a low-power blue laser that releases a 488-nm-wavelength light. The microscopic image is constructed based on this reflected light from the tissue. Before the examination, pretreatment consisting of the topical (cresyl violet or acriflavine) or systemic application (fluorescein) of fluorescence agents is required [39]. One of the main differences between CLE and endocytoscopy is imaging plane depth. While endocytoscopy provides the very superficial mucosal layer with up to 50-μm depth due to the contact light microscopy technique, CLE allows a deeper tissue analysis with up to 250-μm depth [40].
Many studies showed a promising role of CLE for the histologic assessment of mucosal inflammation in IBD. Colonic crypt tortuosity, an enlarged crypt lumen, microerosions, hypervascularization, and augmented mononuclear cell infiltrates were the main findings observed by CLE in active CD [41]. CLE could detect mucosal pathologic abnormalities such as impaired and distorted crypt regeneration, persistent inflammation, and abnormal vascular patterns in UC patients with normal mucosa on white-light endoscopy [42]. More importantly, clinical relapse in IBD could be predicted based on CLE analysis. A composite score using fluorescence leakage and crypt diameter detected by CLE predicted clinical relapse of UC in the following 1-year period [43]. Another study revealed that ileal fluorescein leakage and microerosions on CLE were significant risk factors for flare-ups in CD [44]. Several studies determined the value of CLE for assessing intestinal barrier function in vivo [45-48]. An increased gap density on CLE in the terminal ileum of patients with IBD was a significant predictor of poor clinical outcomes including flare-ups, hospitalization, or surgery in IBD [45,48].
A recent study provided another encouraging role of CLE for molecular imaging in vivo [49]. In 25 patients with CD, the topical administration of a fluorescent anti-tumor necrosis factor (TNF) antibody was used to detect mucosal cells expressing membrane-bound TNF during CLE. Surprisingly, there was a significant difference in clinical response rates at week 12 after adalimumab therapy between patients with CD with many TNF-expressing cells and those with few or no TNF-expressing cells (92% vs. 15%, p=0.0002). This result suggests a new potential of CLE in the field of molecular imaging for identifying therapeutic responses to biologics in IBD.
Regarding the detection of neoplasia in IBD, studies have failed to show a consistent benefit of CLE [50-52]. Compared with NBI, CLE showed significantly low sensitivity for the diagnosis of neoplasia during surveillance colonoscopy in 22 patients with UC [53]. A prospective study evaluating the diagnostic accuracy of CLE for detecting neoplastic lesions in 61 patients with CD reported similar accuracies of dye CE alone and dye CE in combination with CLE (80.3% vs. 86.7%) but poor sensitivity in both groups (42.9% vs. 28.6%) [52]. It concluded that CLE had limited applicability, mainly due to frequent equipment failure. This failure of CLE as a surveillance strategy might be explained by several factors. First, the entire gastrointestinal tract cannot be covered by endomicroscopy because it only sees a limited field of view. Therefore, it is necessary to use other macroscopic techniques like CE for finding suspicious areas and targeted biopsy before using endomicroscopy. Second, it is not applicable for pedunculated or sessile lesions. And finally, high cost, long procedural time, and the need for additional equipment are obstacles to CLE use. More studies are needed to confirm the efficacy of CLE in daily practice.
CONCLUSIONS
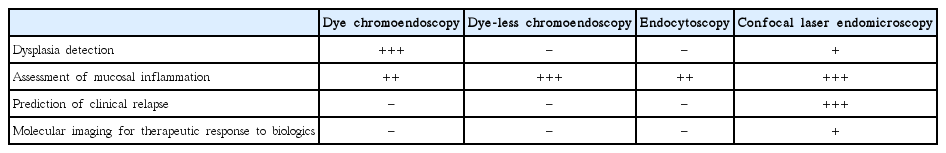
The value of endoscopy in the management strategy of IBD is increasing. In the meantime, advanced endoscopic imaging technologies have almost revolutionized the role of endoscopy in IBD (Table 1). Dye CE is recognized as the gold standard tool for dysplasia surveillance in long-standing IBD. Dye-less CE approaches like NBI play a crucial role in the assessment of mucosal inflammation extent and severity in IBD. Endocytoscopy and CLE enable the real-time histologic evaluation of intestinal mucosa in vivo. CLE can reliably predict clinical relapse in patients with quiescent IBD. Further, CLE can be used for molecular imaging in the prediction of therapeutic response to biologics in IBD, indicating the potential of personalized medicine. The long-term efficacy, feasibility, and cost-effectiveness of these advanced techniques in daily routine practice remain to be discussed.
Notes
Conflicts of Interest:The author has no financial conflicts of interest.