INTRODUCTION
Anisakis simplex is a nematode belonging to the order Ascaridida, family Anisakidae, and subfamily Ascaridoidea. Any fish or cephalopod species can be parasitized by third-stage Anisakis larvae. Codfish, hake, sardines, anchovies, salmon, tuna, and squid are among the most frequently parasitized species. Humans acquire infection by the ingestion of third-stage Anisakis larvae.1,2 Invasive anisakiasis occurs when larvae are attached to or embedded in, or penetrate the host tissues. Anisakis spp. are most often implicated in this type of anisakiasis. They have been found in the mucosa or submucosa of the stomach and intestine and migrate to other tissues such as the omentum, pancreas, liver, and probably the lung.3-5 Van Thiel et al. reported the first case of anisakiasis, in the Netherlands in 1960. Subsequently, many cases have been reported in Japan and Western Europe, where raw fish are consumed frequently. Thereafter, several patients suffering from acute anisakiasis and mainly showing gastrointestinal symptoms such as abdominal pain, nausea, vomiting, and diarrhea have been analyzed via gastroduodenoscopy. There have been many reports on gastric anisakiasis, but only a few reports on colonic anisakiasis. There are also a few reports about endoscopic ultrasonography on gastric anisakiasis and no report about endoscopic ultrasonography on colonic anisakiasis. We report a case of anisakiasis invading the stomach and the colon at the same time after eating anchovies with endosopic utrasonographic findings and biopsy findings.
CASE REPORT
A 47-year-old male visited the hospital for abdominal pain lasting for 3 days. He was diagnosed with acute appendicitis 10 years ago and had an appendectomy operation. He did not have a medical history of hypertension or diabetes mellitus. He was an ex-smoker and social drinker. In the history taking, he was found to have eaten raw anchovies 4 days ago. Vital signs at admission were measured as blood pressure 120/70 mm Hg, pulse rate 68/min, respiration rate 16/min, and body temperature 37Ōäā. There was no urticaria or no facial angioedema. There was no splenomegaly or palpable mass from abdominal palpitation. There was epigastric and periumbilical tenderness but no rebound tenderness. Digital rectal examination was negative. Complete blood count result showed hemoglobin 14.2 g/dL, white blood cell 10,350/mm3 (eosinophil 1.6%), platelet 221,000/mm3, and erythrocyte sedimentation rate 4 mm/hr. Blood chemistry was analyzed as total bilirubin 0.9 mg/dL, aspartate aminotransferase 29 IU/L, alanine aminotransferase 35 IU/L, gamma glutamyl transpeptidase 68 IU/L, alkaline phosphatase 138 IU/L, lactate dehydrogenase 351 IU/L, blood urea nitrogen 20.2 mg/dL, creatinine 1.0 mg/dL, Na 142 mmol/L, K 4.0 mmol/L, Cl 109 mmol/L, amylase 15 IU/L, lipase 32 IU/L, and C-reactive protein 0.34 mg/dL. Urinalysis was normal. Gastroscopy was performed for the suspected acute gastritis.
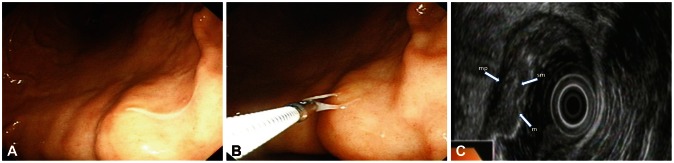
The gastroscopic finding showed that there was a marked mucosal swelling and one thread like living worm (about 1.0 to 1.2 cm) was found penetrating the gastric mucosa in the lesser curvature of the lower body (Fig. 1A). It was withdrawn with biopsy forceps and biopsy was done (Fig. 1B). For the mucosal swelling lesion, endoscopic ultrasonography was performed to rule out the possibility of a submucosal tumor. The endoscopic ultrasonographic finding showed that there was diffuse mucosal and submucosal swelling without mass (Fig. 1C). The most affected layer was the submucosa. Its character was homogenous and relatively hypodense. The 5-layered wall structure was well preserved. After gastroscopy, patient's abdominal pain decreased but still remained. Colonoscopy was performed for the evaluation of the remaining symptom.
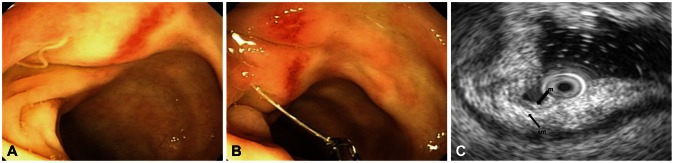
The colonoscopic finding showed that there was round erythematous mucosal swelling and one thread like living structure (about 1.0 to 1.1 cm) was found penetrating the colonic mucosa in the transverse colon area (Fig. 2A). It was also withdrawn with biopsy forceps and biopsy was done (Fig. 2B). The endoscopic ultrasonographic finding showed that there was diffuse submucosal swelling (Fig. 2C). Thickened submucosal layer showed generally homogenous echo and was not easily distinguishable from other layers.
After endoscopic extraction of the living worm, the patient's symptoms were relieved. In order to eradicate Anisakis larvae that might be in small intestine, we prescribed albendazole (400 mg) twice daily for 14 days. Two days later, a follow-up gastroscopy was performed. There was no living structure observed and the gastric mucosa swelling was markedly improved. The stomach biopsy result was gastritis with eosinophil infiltration without eosinophilic granuloma (Fig. 3A). The colon biopsy result was colitis with eosinophil infiltration (Fig. 3B).
The patient was discharged and now is followed up as an outpatient.
DISCUSSION
Anisakiasis is one of the fish-transmitted infection that results from accidental ingestion of third-stage larvae belonging to the family Anisakidae.1-3 Infestation with A. simplex causes direct damage to the tissues followed by the invasion of the gut wall, the development of an eosinophilic granuloma, the perforation of the gut, and strong allergic reactions.1,3 Allergic manifestations range from urticaria to anaphylaxis.1,3 Anisakiasis is relatively common in Korean due to their eating habits. The incidence of gastric anisakiasis is associated with consumption of raw fish. Most patients have severe epigastric pain, which can begin within hours of ingestion of the parasite.2,6 The clinical symptoms of anisakiasis are classified into acute and chronic infections.2,7
Acute infection occurs by third stage larvae invasion of the stomach or the intestine. Symptoms develop due to direct larval invasion into the mucosa of the stomach or hypersensitivity to the larvae or its secretions.1-3,7 The symptoms are characterized by epigastric pain, nausea, and vomiting.
Chronic anisakiasis results from an invasion of larvae into the mucosa or submucosa, and causes abscesses or eosinophilic granulomas with fever, eosinophilia, diarrhea, and abdominal pain. It can mimic appendicitis, gastroduodenal ulcer, inflammatory bowel diseases, or intestinal obstruction.2,3,8
The pathogenesis of gastric anisakiasis is considered to include an allergic reaction to the Anisakis antigen. When the larva of Anisakis dies after penetrating the mucosa, eosinophil infiltration, and proliferation of the connective tissue occur around the larval body, followed by formation of an eosinophilic granuloma, which has the appearance of a submucosal tumor. Subsequently, the granuloma usually decreases in size and gradually disappears.3,9,10 In this case, eosinophilia was absent but eosinophil infiltration was observed.
Endoscopic findings are variable. Besides directly visualizing worms penetrating into the gastric mucosa, endoscopy may reveal erythema, edema, erosive, hemorrhagic gastritis, tumor, or ulceration.6,10,11
Endosopic ultrasonography may reveal that the main inflammatory lesion in anisakiasis is in the submucosal layer (third layer with low echoic changes) of the gastric wall.12,13
Although the echo level of the thickened submucosal layer is relatively low at the penetration site, the internal echo of the thickened submucosal layer is generally homogenous and shows a fine lamellar structure. These endoscopic ultrasonographic findings are the characteristics of the anisakiasis.12,13 There is no report of colon endoscopic ultrasonography for anisakiasis worldwide, but our results show that there was submucosal swelling in the colon. Thickened submucosal layer showed generally homogenous echo and was not easily distinguishable from the other layers.
Diagnosis of anisakiasis can be made by history taking of raw marine fish eating and by discovery of anisakid larvae through endoscopy or surgery.2,6,8,9,12-14
Anisakiasis can be misdiagnosed as peptic ulcer, gastritis, or gastric tumor. Due to nonspecific symptoms associated with intestinal infections, the differential diagnosis is broad and includes appendicitis, ileitis, diverticulitis, eosinophilic gastroenteritis, cholecystitis, colonic tumor, and inflammatory bowel disease.2,15 Infected persons may be diagnosed at the time of appendectomy for presumed appendicitis, and intestinal obstruction from anisakiasis can mimic other obstructive lesions.15 Endoscopic extraction is the best treatment for anisakiasis. Albendazole treatment for anisakiasis is controversial. Some papers reported the effectiveness of albendazole medication on anisakiasis.16,17
Best prevention against anisakiasis is to avoid eating raw fish.2,6 Thorough cooking and adequate freezing of seafood are easy preventive measures against infection with anisakid nematodes.2,18,19
If Anisakiasis is suspected through patient's medical history and physical findings, early endoscopic diagnosis and treatment are the best way to avoid unnecessary test and surgery.