Introduction
Tumor embolism is a rare cause of organ ischemia, with lungs being the most common site. Diagnosing tumor embolism is also difficult as symptoms can be very nonspecific. Ischemia of the rectum with resultant perforation from tumor embolism is very rare. To our knowledge, this is the first case reported.
Case report
A 42-year-old woman with no previous medical history presented to the gynecology clinic with a 2-month history of left iliac fossa pain and worsening left lower limb swelling, associated with loss of weight and appetite. The CA-125 level was increased at 118 U/mL (reference range, 0ā35 U/mL), with a normal carcinoembryonic antigen level of 2 U/mL (reference range, 0ā5 U/mL). Computed tomography (CT) of her abdomen and pelvis showed a 10Ć5 cm left ovarian mass, likely malignant in nature, with tumor deposits at the left iliac and pelvic sidewall, lymph nodes, omentum, peritoneum, and left psoas muscle. The tumor mass extrinsically compressed the left iliac vein. Doppler ultrasonography confirmed complete acute thrombosis of the left external iliac vein. She was started on subcutaneous low molecular weight heparin for a period of 3 months for treatment of deep vein thrombosis.
She underwent total abdominal hysterectomy, pelvic lymph node dissection, excision of left psoas muscle tumor, partial pelvic peritonectomy, infracolic omentectomy, and double-J stent insertion in her left ureter, 3 months after initial presentation. Enoxaparin was stopped 1 day prior to surgery, and an internal vena cava filter was inserted just prior to surgery.
Intraoperatively, a moderate amount of ascites with caking of the omentum was observed. The left ovarian mass was approximately 8 cm in size, with tumor deposits in the right ovary, uterus, and peritoneum and a necrotic lymph node adjacent to the left psoas muscle. There was extensive disease seeding on the small and large bowel mesentery, but no obvious invasion of the rectum. The left ureter was also dilated proximally due to tumor compression. The surgery was largely uncomplicated with no significant hemostasis or coagulation issues. A Jackson-Pratt drain was inserted in the pouch of Douglas.
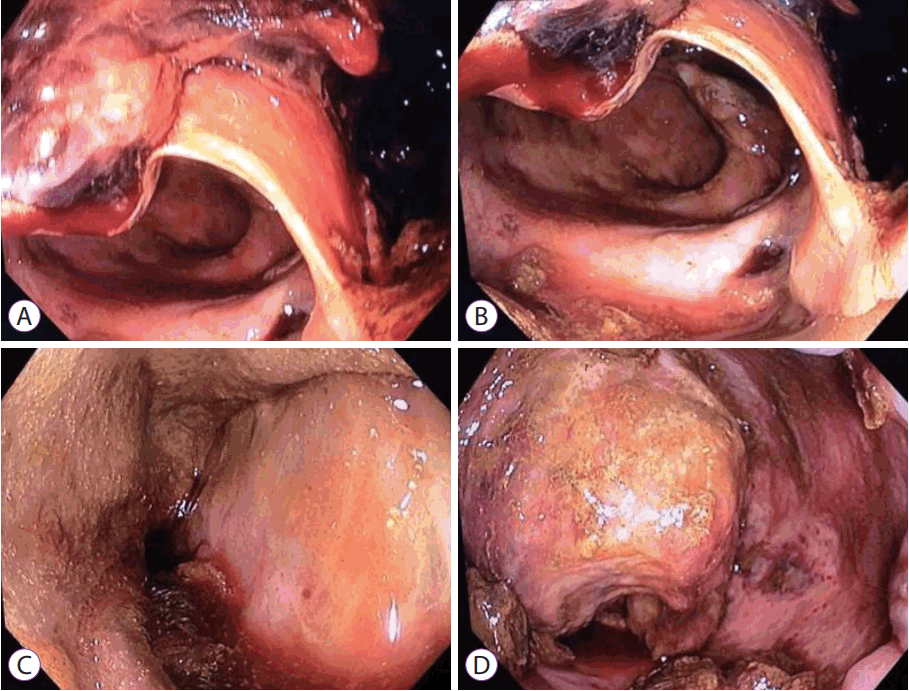
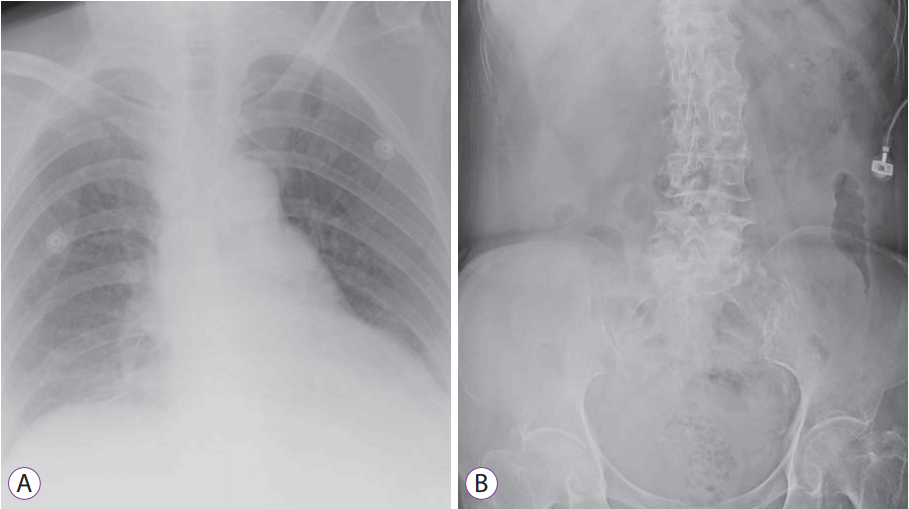
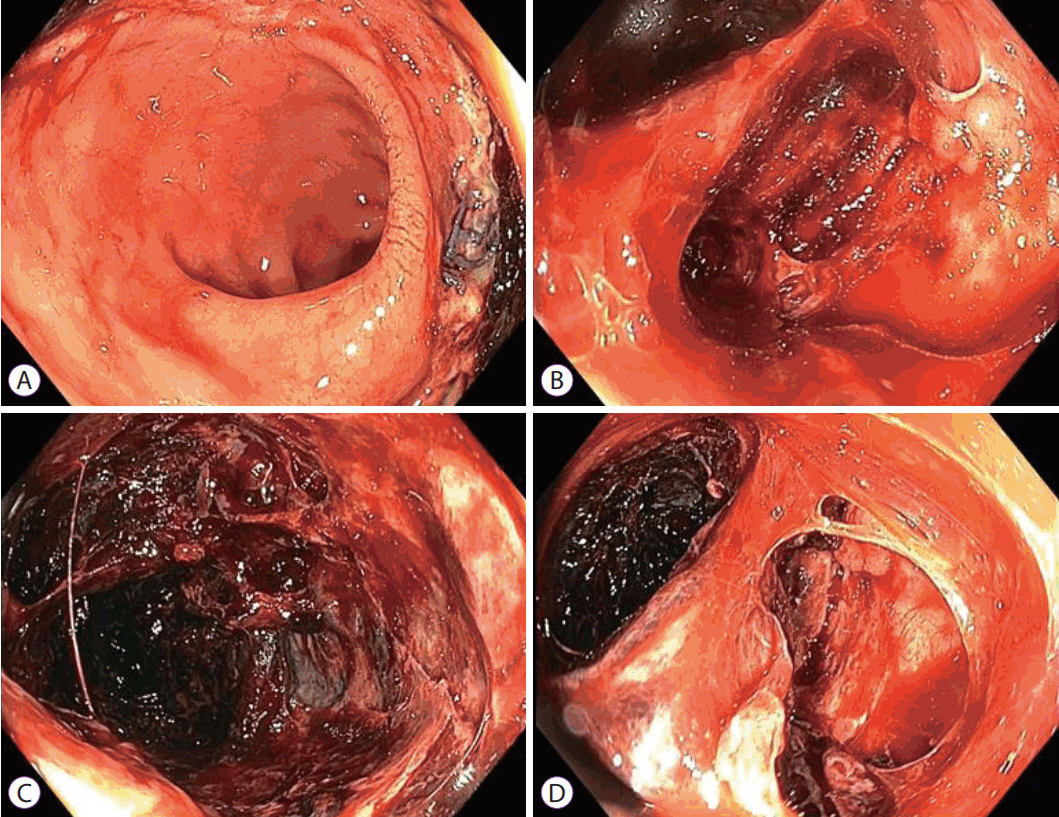
She developed febrile episodes with loose stools and rectal bleeding a few days later. Flexible sigmoidoscopy performed on postoperative day 7 showed a large mucosal defect at the right posterolateral rectal wall with extensive mucosal flap dissection at about 15 cm from the anal verge. The mucosal flap was ischemic in appearance, but there was no evidence of full-thickness rectal infarction (Fig. 1). Chest and abdominal X-ray showed no pneumoperitoneum. On review of the preoperative CT scan, the inferior mesenteric artery as well as the rectum showed appropriate contrast enhancement. She was managed conservatively with intravenous antibiotics as X-rays did not show evidence of perforation (Fig. 2). However, she developed abdominal pain with fresh rectal bleeding approximately a week later. Repeat sigmoidoscopy showed large amounts of old and fresh blood clots within the rectum, with extensive necrosis of the rectal mucosa. The previous mucosal flap had developed into a defect in the anterior rectal wall where the vaginal vault could be visualized (Fig. 3). A CT mesenteric angiogram confirmed the rectal wall defect, showing communication with the apex of the vaginal vault, and apparent fecal material. There was no evidence of active contrast extravasation (Fig. 4).
Repeat laparotomy on postoperative day 17 revealed a perforation in the ischemic anterior rectal wall with dehiscence of the vaginal vault. A Hartmanās procedure was performed in which the rectum was resected to the level of the defect and over-sewn, the vaginal vault was closed primarily, and a sigmoid colostomy was created.
Histopathologic examination of the initial specimen showed a high-grade, poorly-differentiated serous carcinoma of bilateral tubal derivation, predominantly involving the omentum, pelvic peritoneum, and both ovaries. Multiple tiny uterine serosal tumor seedlings and lymphovascular tumor involvement were also seen. Native endometrium was in the late secretory phase. A 9-cm rectal specimen showed a hemorrhagic anterior surface and pale fibrotic areas within the perirectal fat. No mucosal tumor was seen. Histologically, the anterior rectal wall, as well as a smaller focus at the distal surgical margin, showed transmural infarction with perforation, all in the absence of any evidence of intramural tumor. Extensive fibro-inflammatory reactive changes with necrosis in the perirectal fat showed multiple scattered accompanying small metastatic foci of high-grade serous carcinoma with vascular emboli (Fig. 5).
The patient remained stable postoperatively and had no further episodes of bleeding. Enoxaparin was restarted, and she was discharged well. She had adjuvant chemotherapy and was followed up for 6 months after surgery.
Discussion
Ovarian carcinoma usually presents at an advanced stage with peritoneal disease in about 70% of cases [1]. In advanced disease, there is direct spread of tumor within the peritoneum and the visceral organs via peritoneal fluid. Such extensive seeding of disease is associated with high-grade carcinoma and is known as carcinomatosis [2]. Patients with such advanced malignancies are at high risk of thromboembolism. Less commonly, tumor embolism can also cause occlusion of vessels and presents similarly to thromboembolism. Pathologically, tumor embolism is defined as a cluster of tumor cells within the arterial system that is not contiguous with locally advanced or metastatic disease, resulting in flow disturbances. As malignant cancer cells also induce local coagulation, vascular obstruction can often be a combination of both thrombus and tumor cells [3].
The most common site of tumor embolism is the lung, as the pulmonary bed receives the entire cardiac output [4]. Patients can present with dyspnea if there is significant obstruction of pulmonary vasculature. However, this is rarely recognized before death due to the difficulty of establishing the diagnosis; the diagnosis is only made in 6% of patients with cancer [5], with autopsy studies showing an incidence of 0.9%ā2.4% [3,6]. An autopsy study found that tumors arising from the lungs, ovaries, kidneys, and liver are associated with a higher incidence of tumor emboli [7]. The majority of tumor emboli reported in case studies are from the breast, stomach, and lung [5].
Various case studies have discussed bronchogenic carcinoma resulting in systemic malignant tumor embolism [8-12]. Tsoi et al. reported a case of breast cancer resulting in multi-organ failure with disseminated tumor emboli to the brain, heart, lungs, colon, spleen, and thyroid [13]. Tumor emboli involving other organs such as the brain, kidneys, or the peripheral limbs are also possible but are much rarer. Jo et al. reported a case of recurrent rectal cancer presenting with an acute abdomen from a perforated terminal ileum due to tumor embolism [14]. Histology from the resected specimen showed extensive tumor emboli with transmural infarction of the ileum, without any discrete tumor mass [14].
In our case, we observed the transition from rectal ischemia with mucosal dissection to full-thickness infarction and perforation through repeated sigmoidoscopies over a period of a week. Histopathologic findings suggested that the rectal perforation in this case was the result of infarction secondary to the presence of numerous microscopic tumor vascular emboli, rather than being due to a tumor mass effect. A propensity for intravascular spread by this carcinoma was also observed in the previous surgical specimen.
We initially elected to treat the rectal mucosal dissection conservatively with intravenous antibiotics and bowel rest, as the patient remained clinically well with no evidence of bowel ischemia or perforation. An alternative approach would have been to proceed directly to laparotomy with bowel resection and stoma creation, which we believed would have been difficult to justify at that stage as the patient was clinically well.
While the majority of tumor emboli are undiagnosed, especially if they are asymptomatic, those presenting with symptoms are usually more severe and require active management. In retrospect, we acknowledge that the mucosal injury from tumor emboli in our case was extensive and that early surgical intervention was warranted. As patients with advanced malignancies are at high risk of tumor embolism, early thromboprophylaxis should be considered. Clinicians should have a high index of suspicion for tumor embolism, as symptoms can vary depending on the organs affected.