INTRODUCTION
Currently, endoscopic procedures with submucosal tunnel forming are widespread, including peroral endoscopic myotomy (POEM), submucosal tunneling endoscopic resection (STER), and tunneling biopsy of submucosal tumors of the gastrointestinal tract.1 This technique can also be applied to access the muscular layer and myenteric plexus of the esophagus for pathological verification in patients with esophageal motility disorders.
The most precise assessment method for obtaining the myenteric plexus and muscular layer specimens is a full-thickness biopsy. The concept and indications of this method are described in the guidelines for histological techniques and reporting, on behalf of the Gastro 2009 International Working Group [2].
In our case, we describe tunneling biopsy of the muscular layer in a patient with systemic sclerosis (SS) for the histological verification of the esophageal involvement in SS.
CASE REPORT
In 2011, a 48-year-old woman had multiple newly found calcifications in the skin of the back, hips, and shoulders. For the next 5 years, they remained stable; however, in 2015, signs of Raynaud syndrome appeared. At the same time, firm plaques of the skin were revealed.
In March 2016, the clinical picture worsened with the appearance of edema of the fingers (тpuffy fingersт). Moreover, skin calcifications were confirmed by radiography. Summarizing all the symptoms mentioned, it was concluded that the patient had CREST syndrome, and treatment with systemic prostaglandins, bisphosphonates, and D-penicillamine was started.
After a 2-year treatment, the calcifications became smaller but Raynaud syndrome remained stable; therefore, bosentan was prescribed.
In November 2018, the patient visited our hospital with complaints of dysphagia. High-resolution esophageal manometry was performed. In the main test, in 100% of swallows, no esophageal contractions were detected (Fig. 1A). The integrated relaxation pressure was normal. CCv3.0 classified such results as major disorders of peristalsis, in particular, absent contractility [3]. During a multiple rapid swallow test, esophageal contractions were not detected (Fig. 1B). In addition, in the beginning of the procedure and after the main test, we measured esophagogastric junction contractile integral (EGJ-CI) to assess the barrier function of the EGJ. In both cases, the EGJ-CI was null, indicating significant hypotension of the EGJ, decreased barrier function, and increased risk of pathological reflux [4].
After 2 days, a submucosal tunneling muscle biopsy was performed:
Twenty-five centimeters from the incisors on the posterior wall with CO2 insufflation, a 1.3-cm initial mucosal incision was made. After the submucosal injection, only slight elevation of the mucosa was detected. After forming the short submucosal tunnel, full-thickness resection of the muscular layer (1.0-cm diameter) was performed using an electrosurgical knife. When a specimen was separated from the underlying tissue, it was evacuated using an endoscopic loop. Initial mucosal incision was closed using endoscopic clips (Fig. 2).
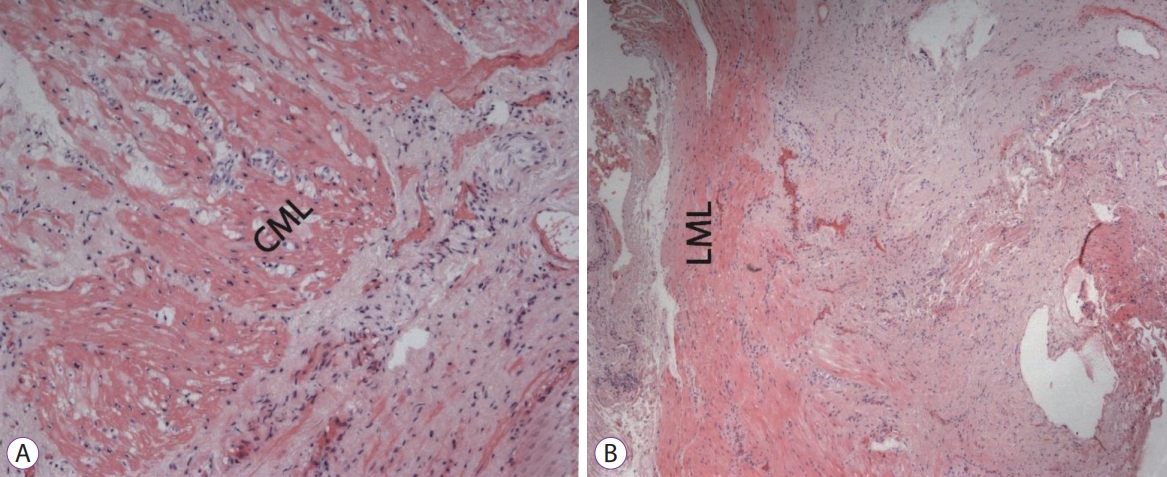
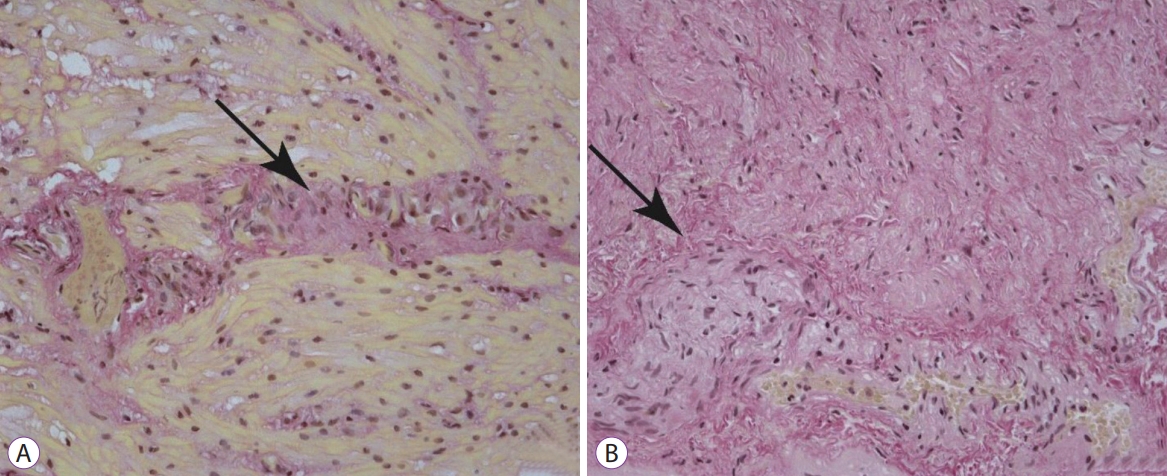
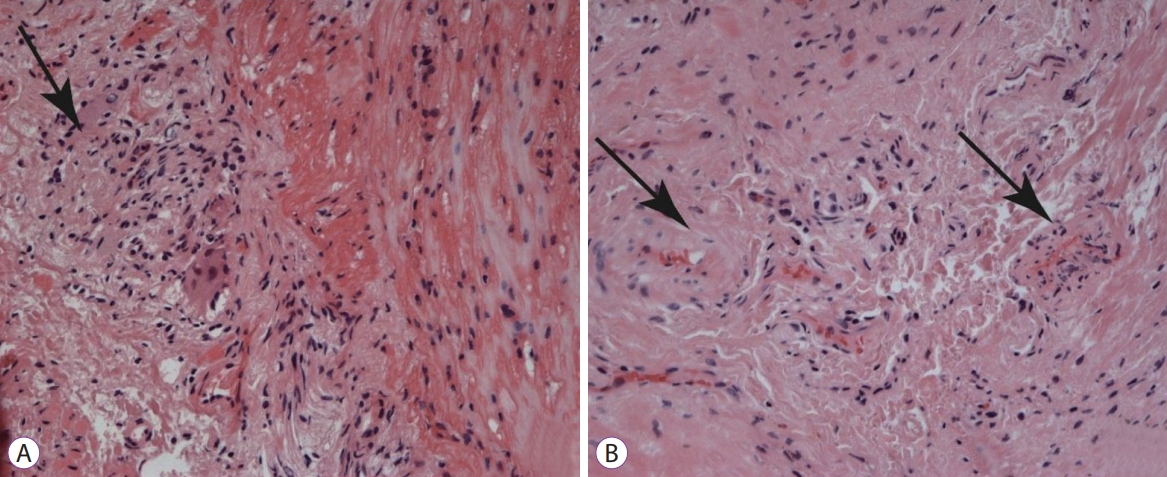
Histological samples were stained with hematoxylin and eosin, picrofucsin (Van Giesonтs technique), Congo red, and Massonтs trichrome. Atrophic changes with diffuse sclerosis were revealed in the longitudinal and circular muscular layers of the esophagus (Fig. 3), as well as perivascular and perineural sclerosis (Fig. 4). In the myenteric plexus, inflammatory infiltration composed predominantly of lymphocytes was observed (Fig. 5A). Microcirculatory vessels were also implicated in the disease, appearing with thickening of the vessel wall, and proliferative endarteritis with obliteration of the lumen of the blood vessels (Fig. 5). Verified progression of the esophageal impairments and persisting skin changes resulted in the replacement of D-penicillamine with mycophenolate mofetil and the addition of iloprost for the treatment of Raynaud syndrome.
On postoperative day (POD) 1, the patient was administered with water. There was no fever and no complaints of pain. On POD 2, the patient was discharged for outpatient follow-up
Three months later during a follow-up esophagoscopy, a 0.5 cm mucosal scar was observed in the location of the initial incision. No complaints from the patient were received.
DISCUSSION
Histological investigation of the fragment of the esophageal muscular layer, obtained by endoscopic tunneling biopsy, contributed not only to the verification of the esophageal involvement and myenteric plexus in SS but also to the severity of pathological changes. Based on that knowledge, we changed the treatment tactics: D-penicillamine was replaced by mycophenolate mofetil.
There are several techniques for the biopsy of the muscular layer of the gastrointestinal tract, but they have some limitations. In endoscopic no-hole full-thickness biopsy, there is no control specimen size, which is vital for the proper evaluation of the myenteric plexus. Myenteric plexus is not homogenous in the entire length of the esophagus and has ganglia at different distances from each other, thus the size of the biopsy material must be sufficient [5,6].
According to the literature, endoscopic ultrasound-guided fine-needle aspiration is a safe and effective method, although there are some limitations: parts of the muscular layer are fragmented and it is difficult to correctly place the obtained specimens for a meaningful histological investigation [7].
Considering the limitations of the techniques mentioned above, we propose the tunneling method as a safe method to perform biopsy with a direct visual control of the region of interest. Nowadays, many medical centers, such as ours, have sufficient experience in POEM and STER, making submucosal tunneling a mastered and routine procedure.
In conclusion, endoscopic tunneling biopsy is a safe and effective method for obtaining specimens of the esophageal muscular layer in patients with esophageal motility disorders to verify pathological changes in the esophageal musculature and myenteric plexus.