INTRODUCTION
Intestinal metaplasia (IM), a well-established premalignant condition of the stomach, is the replacement of the gastric mucosa by epithelial tissue that resembles the intestinal mucosa.1 Two main subtypes of IM have been identified according to their histologies, but IM can also be subdivided into types I, II, and III according to the mucin expression pattern.2 IM type III is often considered an indicator of the future development of the intestinal form of gastric cancer by Lauren's classification.3,4 However, there is still a debate on the sequential progression of IM from type I to type III via the type II intermediate.
Human trefoil peptides (TFFs) and mucin glycoproteins are expressed predominantly in the gastrointestinal tract, play key roles in mucosal protection by forming a mucous barrier, and regulate mucosal repair by promoting recovery after injury.5 Among these peptides and glycoproteins, TFF1 and MUC5AC are mainly expressed in the gastric mucosa and are considered to be gastric-specific types. We previously reported that there is progressive loss of TFF1 and TFF3, together with induction of TFF2, during the development of IM from type I to type III via the type II intermediate.6 But it was limited only to the TFFs. The expression of TFFs is closely related to the expression of mucin glycoproteins5 and we need further investigation of their relationship to clarify the progression pattern of IM subtypes.
We hypothesized that the expression of gastric-specific TFFs and mucin glycoproteins may change in a specific manner when IM progresses from type I to type III via the type II intermediate. The aim of this study was to identify the sequential progression pattern of IM subtypes by analyzing the expression patterns of TFF1 and MUC5AC.
MATERIALS AND METHODS
Subjects
The study protocol was approved by the Institutional Review Board of The Catholic University of Korea and written informed consent was obtained from all participants. This study was performed from March 2003 to September 2003, at Incheon St. Mary's Hospital, The Catholic University of Korea. Endoscopic biopsies were performed in patients with non-ulcer dyspepsia after methylene blue chromoscopy. Two specimens were taken from the corpus, and another two from the antrum. Each specimen was fixed in 10% formalin and was routinely embedded in paraffin wax. Serial sections were cut and used for histochemistry and immunohistochemistry analyses.
Staining for IM subtypes
Alcian blue (pH 2.5)/periodic acid-Schiff staining and the high iron diamine-alcian blue technique were used to identify neutral, sialomucins, and sulfomucins.7 IM status was considered positive when at least one specimen was positive in alcian blue/periodic acid-Schiff staining. IM was classified according to Filipe and Jass2 as follows: type I, mature absorptive cells and goblet cells, the latter secreting sialomucins; type II, few or no absorptive cells presence of columnar intermediate cells in various stages of differentiation secreting neutral and acid sialomucins and goblet cells secreting sialomucins or, occasionally, sulfomucins, or both; and type III, columnar intermediate cells secreting predominantly sulfomucins, and goblet cells secreting sialomucins or sulfomucins, or both. When subtypes are mixed in one or two specimen from the same locus, the main type was analyzed.
Immunohistochemistry of MUC5AC and TFF1
MS-145-P (Neomarkers, Fremont, CA, USA), a monoclonal antibody against MUC5AC, was used. In brief, formalin-fixed 5 ТЕm sections were deparaffinized, rehydrated, and washed in tris-buffered saline (TBS). Endogenous peroxidase activity and nonspecific binding were blocked by incubation with 3% hydrogen peroxide (H2O2) for 5 minutes, and blocking solution (normal immune serum) was added for 10 minutes, followed by the MUC5AC primary antibody (dilution 1:100) for 30 minutes at 40т. After rinsing with TBS, alkaline phosphatase was added, and the slides were incubated for 15 minutes at 45т and then rinsed again with TBS. Fast red chromogen was added, and the samples were incubated for 15 minutes at 45т. Then, contrast staining was performed using Mayer's hematoxylin stain.
A mouse monoclonal antibody (kindly provided by Drs. N. Wright and G. Elia, Cancer Research UK, London, UK) was used for TFF1 immunohistochemistry as described in our previous study.6
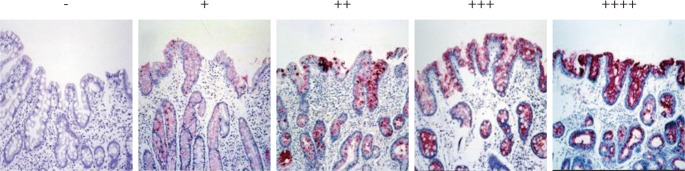
The immunoreactivity of MUC5AC and TFF1 was graded from 0 to (++++) according to the percentage of positive cells: 0 for no positive epithelial cells, (+) for fewer than 25% of the epithelial cells, (++) for 25% to 50% of the epithelial cells, (+++) for 50% to 75% of the epithelial cells, and (++++) for more than 75% of the epithelial cells. Two pathologists reviewed the results and discussed instances where their classification did not agree.
RESULTS
IM patterns
Endoscopic biopsies were performed in 80 patients, and endoscopic IM was found in the corpus in 50 patients and in the antrum in 78 patients. In the corpus, type I IM was found in 24 patients (48%), type II in 14 patients (28%), and type III in 12 patients (24%). In the antrum, type I IM was found in 24 patients (30.8%), type II in 23 patients (29.5%), and type III in 31 patients (39.7%). Type III IM was more frequently found in the antrum than in the corpus (p=0.03).
Immunohistochemical reactivity to MUC5AC and TFF1
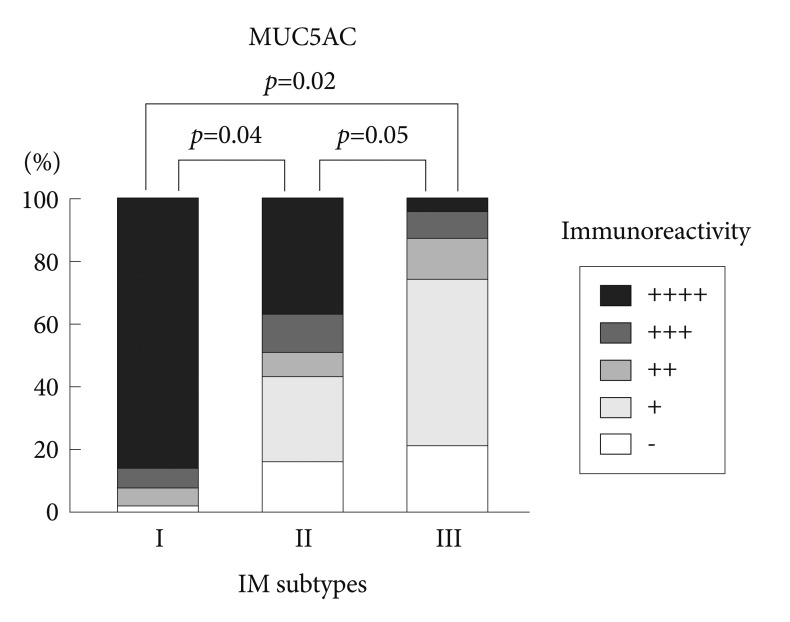
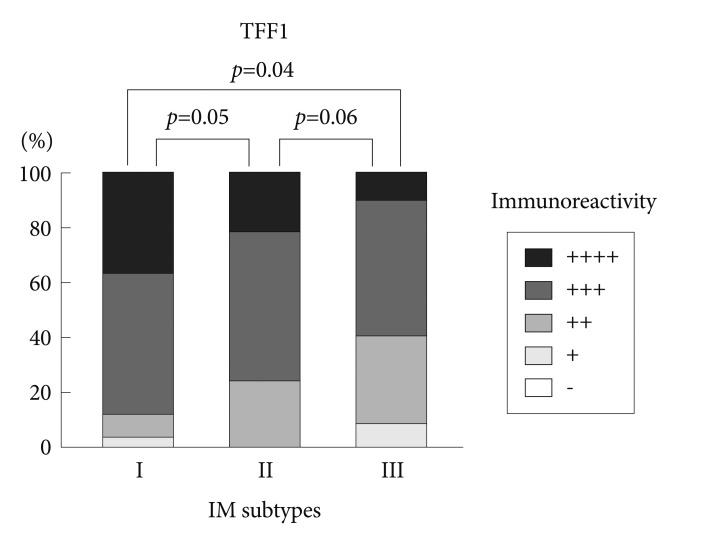
Immunohistochemical reactivity to MUC5AC decreased during the progression from type I to type III both in the corpus and in the antrum (Fig. 1). MUC5AC was mainly expressed in columnar epithelial cells. Examples of immunohistochemical reactivity to MUC5AC are presented in Fig. 2. Immunohistochemical reactivity to TFF1 also decreased during the progression from type I to type III both in the corpus and in the antrum (Fig. 3). TFF1 was mainly expressed in foveolar cells. Examples of immunohistochemical reactivity to TFF1 are presented in Fig. 4.
DISCUSSION
Mucin is a glycoprotein containing many glycosyl groups. Mucin is a major component of mucus and covers the epithelial cells of the gastrointestinal tract. Approximately 13 kinds of mucins have been identified and can be classified as secretory or membrane-associated types according to their relationship to epithelial cells. These mucins contribute to the defense of the gastric mucosa.8 In the gastric mucosa, MUC1, MUC3, MUC4, MUC5AC, MUC5B, and MUC6 have been identified, and MUC5AC is a mainly expressed mucin.8 The process of neoplastic transformation in the stomach has been known to be associated with decreased expression of the mucins that are normally expressed in gastric mucosa, including MUC5AC.9
TFFs are well known to be associated with the expression of mucins and also play important roles in the defense and regeneration of gastric epithelia.10 Three types of TFF have been identified, namely TFF1/pS2, TFF2/SP, and TFF3/ITF. TFF1 is expressed mainly in the gastric mucosa, and decreased expression of TFF1 in gastric cancer has been reported.11,12
TFF1 is co-expressed with the secreted mucin MUC5AC in superficial cells of the gastric mucosa, and a direct association between TFF1 and MUC5AC has been reported.13 In another study, TFF1 and MUC5AC were similarly expressed in gastric carcinomas,14 a result that suggests that decreased co-expression of TFF1 and MUC5AC may play a role in gastric carcinogenesis. In this study, we found that the expression levels of MUC5AC and TFF1, a major mucin and major TFF in the gastric mucosa, respectively, gradually decrease during the progression of type I to type III IM. Considering the decrease in the expression of MUC5AC and TFF1 in the progression of Helicobacter pylori-infected pre-neoplastic lesions to gastric adenocarcinoma, this is one form of evidence that type I IM sequentially progresses to type III via type II in gastric carcinogenesis. It is plausible that the decrease in the major gastric-type mucin and TFF makes the cells much more susceptible to damage from other carcinogens and may finally lead to gastric cancer. This should be elucidated in further studies.
Filipe and Jass2 subdivided IM into three types according to the expression of sialomucin and sulfomucin. Among the types of IM, type III IM was reported to be associated with gastric cancer, but the exact mechanism has not been identified. Type III IM was more frequently found in the antrum than in the corpus in the present study. This result suggests that IM begins in the antrum and progresses to the corpus. This progression can be easily explained by the migration of H. pylori from the antrum to the corpus and finally to the cardia. There is a still debate about the relationship between type III IM and gastric cancer,1,15,16 but considering the gradual decrease in the major gastric-type mucin and TFF observed in this study, type III IM might be associated with the development of gastric cancer. Biopsy is mandatory to identify type III IM in a screening endoscopy.15
In conclusion, IM sequentially progresses from type I to type III via the type II intermediate. The downregulation of the major gastric-type mucin MUC5AC and TFF1 might be associated with gastric carcinogenesis. Further studies, including experiments in which these events are interrupted to block the progression of IM and the ensuing gastric cancer, are anticipated.