Two Cases of Russell Body Gastritis Treated by Helicobacter pylori Eradication
Article information
Abstract
Russell body gastritis was first defined in 1998, but not many cases have been reported since then. The exact causes and process of this condition are unknown yet; however, considering the reported cases, it has been highly suggested to have correlation with Helicobacter pylori infection. Russell body gastritis has a non-specific clinical presentation of gastritis such as gastric mucosal edema in the macroscopic view. It can be mistaken as xanthoma, signet ring cell carcinoma, or a malignant lymphoma including mucosa-associated lymphoid tissue lymphoma and plasmocytoma. Russell body gastritis features polyclonal immunoglobulin and is differentiated from Mott cancer, of which immune globulin has monoclonal aspect. Authors report here two cases of Russell body gastritis with examined endoscopic findings as well as a review of related literature on the association of all reported cases of Russell body gastritis with H. pylori infection.
INTRODUCTION
Russell body gastritis is a very rare disease where lamina propria of gastric mucosa is excessively infiltrated by plasma cells containing Russell bodies, which are eosinophilic intracytoplasmic inclusions. Tazawa and Tsutsumi1 first described this benign disease as Russell body gastritis. In South Korea, Yu et al.2 first reported a similar case in 1987, which was not identified as Russell body gastritis at that time. A variety of case reports suggested that Russell body gastritis was associated with Helicobacter pylori infection1,3-7 and that the lesions disappeared after its eradication.1,4,6,7
In this case report, we report two patients who were diagnosed as both Russell body gastritis and H. pylori infection along with a review of related literature.
CASE REPORTS
Case 1
A 57-year-old male, who visited the hospital for a regular check-up, reported intermittent non-severe epigastric soreness, mostly pre-prandial, which subsided post-prandially. His two older brothers died of colon cancer and another older brother of hepatocellular carcinoma.
The patient had a history of colonoscopic polypectomy in 2007 for multiple gastro-colic polyps. During the upper gastrointestinal endoscopy performed in 2007, multiple raised erosions and atrophic gastritis, as well as a gastric polyp on fundus and three polyps on antrum were detected. Moderate H. pylori infection was confirmed by biopsy and light microscopic findings. A follow-up upper gastrointestinal endoscopy, performed in 2008, revealed two hyperplastic polyps, raised erosions and atrophic gastritis on antrum and lower body. He had no smoking history.
At the visit, blood pressure was measured 140/80 mm Hg, pulse rate 80 times/min, respiration rate 20 times/min, and body temperature 36.5℃. In physical examinations, he showed soft abdomen and normal bowel sound, without tenderness or rebound tenderness. In laboratory findings, all measures of peripheral blood test were normal, with hemoglobin 14.5 g/dL, hematocrit 41.7%, platelet 21,600/mm3, and leukocyte 6,310/mm3. Total protein was 7.4 g/dL, albumin 4.4 g/dL, AST 19 IU/L, ALT 15 IU/L, total bilirubin 0.9 mg/dL, BUN 15.3 mg/dL, and creatinine 1.2 mg/dL, without any remarkable finding on urinalysis and chest radiography.
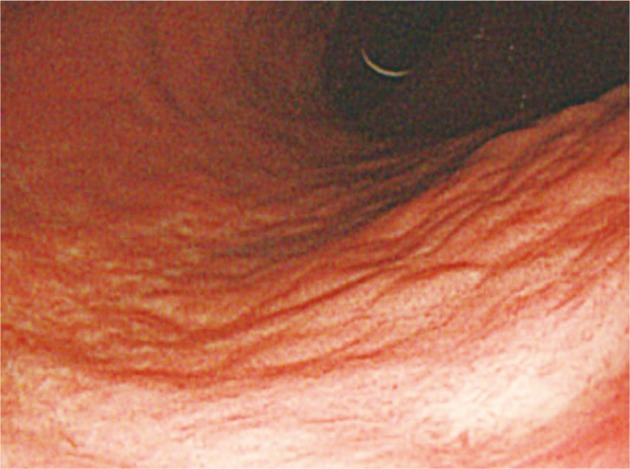
Upper gastrointestinal endoscopy and colonoscopy were performed to detect multiple polypoid lesions, 0.5 to 1 cm in diameter, on lower body of stomach and antrum, and a relatively distinct slightly raised white flat lesion, 2 cm in diameter, on mid-body posterior wall (Fig. 1). This lesion showed nodular morphology on the surface and mild depression in the center. Campylobacter-like organism test was positive. Colonoscopy revealed no specific finding except for internal hemorrhoid.
Upper gastrointestinal endoscopic findings of case 1. Endoscopic image shows whitish elevated lesion on mid-body posterior wall of the stomach.
Biopsy from the lesion on the posterior wall of mid-body revealed lamina propria being infiltrated by plasma cell with abundant eosinophilic cytoplasm (Fig. 2). Materials such as hyaline globule were found in the cytoplasm and stroma (Fig. 2). Cytokeratin staining was negative, and all immunoglobulin on immunohistochemial staining was positive both on kappa and lambda light chains (Fig. 3), suggesting polyclonal nature, which led to the diagnosis of Russell body gastritis.
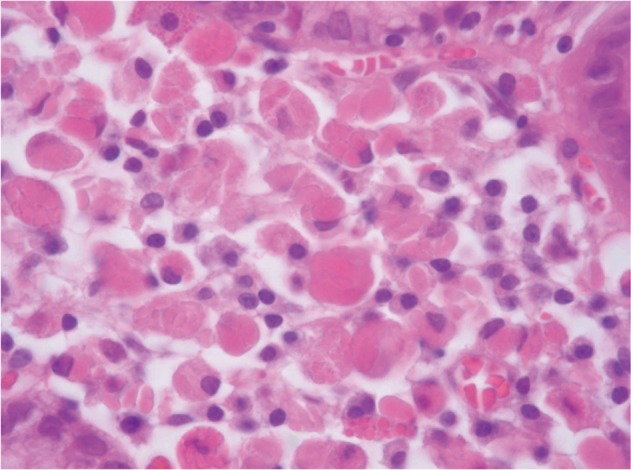
Microscopic findings (H&E stain, ×400). A gastric biopsy showing dense infiltration of plasma cell with abundant eosinophilic cytoplasm in the lamina propria. In the cytoplasm and stroma, dense hyaline globule like materials (Russell bodies) are seen.
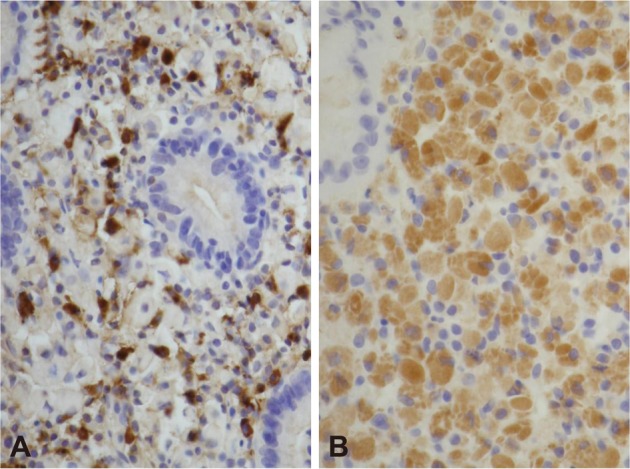
Immunohistochemical stains. The infiltrating cells are positive for immunoglobulin kappa (A, ×200) and lambda (B, ×200) light chain.
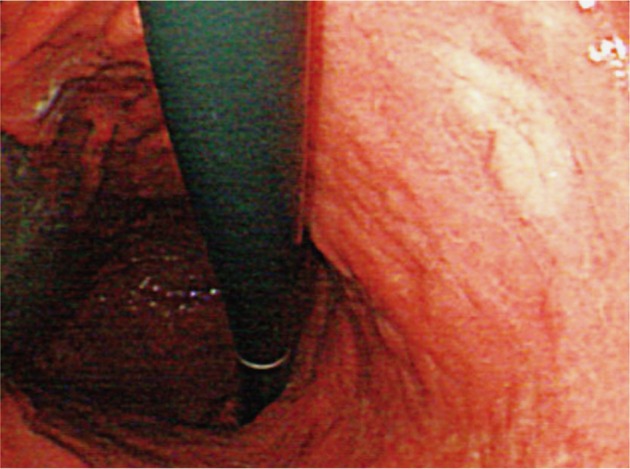
The patient received H. pylori eradiation therapy for 2 weeks with oral lansoprazole 30 mg, clarithromycin 500 mg, and amoxicillin 1,000 mg, 2 times a day. The gastroscopy, performed 3 months after the eradication, revealed the previous lesions were cleared with only mildly-depressed mucosal atrophic discoloration left (Fig. 4).
Case 2
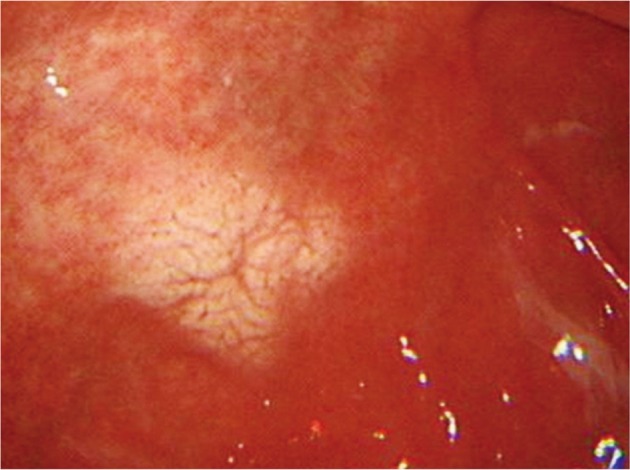
A 43-year-old male patient visited the hospital for a regular check-up. The patient had no specific symptoms, past medical history, or familial history. He was a never-smoker but had a mild drinking history. Chest radiography and laboratory tests revealed no specific finding. In gastroscopy, a whitish oval shaped flat lesion, 2 cm in diameter, was detected on the posterior wall of antrum. The lesion had nodular appearance in general, with slight depression in the center (Fig. 5). The findings of biopsy revealed, as in case 1, infiltration by plasma cells with abundant eosinophilic cytoplasm containing Russell bodies and infection with H. pylori. The findings of immunohistochemial staining were positive both on kappa and lambda light chains, leading to the diagnosis of Russell body gastritis. H. pylori eradication therapy was performed, and no symptom has been observed since then. The gastroscopy, performed 2 months after the eradication, revealed the previous lesions were cleared with only discoloration left.
DISCUSSION
Russell bodies, first reported by Russell in 1890, refer to the immunoglobulin-containing inclusions, made by abnormal secretion of plasma cells, and characterized by the distended rough endoplasmic reticulum. These plasma cells with Russell bodies are called Mott cells,8 which may be observed in epithelial cells of gastrointestinal mucosa with chronic inflammation as well as in multiple myeloma, malignant lymphoma, Hashimoto's thyroiditis, rheumatoid arthritis, ulcerative colitis, and some malignant cells of plasmocytoma.8,9 These Mott cells with poly-clonality are deposited intensively in the stomach, which was first named as Russell body gastritis by Tazawa and Tsutsumi1 in 1998. Plasma cells containing Russell bodies are frequently detected in gastric mucosa of Korean population, but not such intensive depositing. Being a very rare disease both in and outside the country, its pathophysiology, epidemiology, endoscopic findings, clinical manifestations, and treatments are not established yet; but it is considered to be highly associated with chronic inflammatory responses to H. pylori infection, as suggested by the reported cases so far.
The authors examined endoscopic findings, locations, association with H. pylori infection, histologic findings, and posteradication responses in all reported cases of Russell body gastritis in and outside the country (Table 1). Most symptoms were non-specific such as epigastric pain, dyspepsia, and nausea. Endoscopic findings revealed non-specific erosions and yellow-white scar ulcers in most cases, and depressed, edematous, or non-specific gastritis findings in some cases. The antrum was most frequently affected, followed by the body, fundus, and cardiac region. From a total of 14 cases, H. pylori was detected in 10 cases (71%), of which 8 cases (80%) were improved after eradication therapy. One case 3 was not evaluated after the eradication. Other findings included esophageal candidiasis, human immunodeficiency virus, alcoholism, monoclonal gammopathy, analgesics abuse, and multiple gastric or colon polyps.
H. pylori colonization stimulates secretion of various cytokines in the gastric epithelial cells, leading to the activation of immune response, which is the recruitment of neutrophils and granulocytes at first, followed by lymphocytes and later by plasma cells, leading to the plasma cell infiltration in the lamina propria, the hallmark of chronic gastritis.4 A lot of researchers have suggested that chronic antigenic stimulation caused by H. pylori infection facilitates plasma cells to overproduce immunoglobulin to make Russell bodies, which are deposited in the plasma cells.9,10 In addition, most cases, including those reported by Eum et al.9 and Paik et al.5 in South Korea, showed improvement of symptoms and lesions after H. pylori eradication.1
Reviews of Russell body gastritis cases, including the two cases in this study, confirmed that many of the cases were infected by H. pylori, and that symptoms and histological findings improved after its eradication, It is commonly accepted so far, therefore, that H. pylori infection may play a role in Russell body gastritis; however, some cases of Russell body gastritis were reported not to show any evidence of H. pylori infection from immunohistochemical staining.11-13
Johansen and Sikjär10 compared the distribution of Russell bodies between malignant lesions and benign gastritis lesions, and reported that Russell bodies were detected more frequently in normal tissues surrounding malignant lesions, which may reflect the possibility of association between malignancy and Russell body gastritis. This study may have greater implication, considering the association between H. pylori and gastric cancer. Recently, it has also been suggested that highly pathogenic H. pylori genotypes vacA and cagA may be associated with Russell body gastritis.13 Overall, it may be possible to consider Russell body gastritis as one of unspecific inflammatory findings in gastric mucosa, a premalignant condition, or a separate disorder.
Russell body gastritis, in general, has similar endoscopic and histological findings with signet ring cell carcinoma, and is subject to be confused with malignant lymphoma, mucosa-associated lymphoid tissue (MALT) lymphoma, and plasmocytoma; however, it could be differentiated from these diseases by using high resolution microscope to confirm morphological features of plasma cells and Russell bodies and immunohistochemical staining to confirm poly-clonality. Russell body gastritis is also similar with gastric xanthoma at endoscopic findings which has white-yellowish nodular or plaque lesion; however gastric xanthoma could be smaller (0.5 to 1.5 cm) in size and more regular in the nodule shape. It must be evaluated by microscopic examination, if the size is larger or the shape is irregular on endoscopic view. Gastric xanthoma can be distinguished by finding lipid islands on microscopic view.
H. pylori infection is associated with various disorders from chronic gastritis to gastro-duodenal ulcer, to malignant tumor, and to MALT lymphoma. Plasma cells containing Russell bodies are frequently detected in gastric mucosa of Korean population. Considering widespread H. pylori infection in Korea, the prevalence of Russell body gastritis could be higher, which warrants greater concern and further investigation.
Russell body gastritis is a very rare disease, characterized by polyclonal immunoglobulin deposition from immunohistochemical staining. The pathogenesis of this condition has not been established yet, but H. pylori is considered to be strongly associated with it in the view of diagnosis and treatment. Other possibilities have been also suggested, including gastric mucosal inflammation, premalignant lesion or a separate disorder. Russell body gastritis is not accurately differentiated from other cancerous lesions by endoscopy, which is why more case reports and studies are needed.
Notes
The authors have no financial conflicts of interest.