Propofol versus Midazolam for Sedation during Esophagogastroduodenoscopy in Children
Article information
Abstract
Background/Aims
To evaluate the efficacy and safety of propofol and midazolam for sedation during esophagogastroduodenoscopy (EGD) in children.
Methods
We retrospectively reviewed the hospital records of 62 children who underwent ambulatory diagnostic EGD during 1-year period. Data were collected from 34 consecutive patients receiving propofol alone. Twenty-eight consecutive patients who received sedation with midazolam served as a comparison group. Outcome variables were length of procedure, time to recovery and need for additional supportive measures.
Results
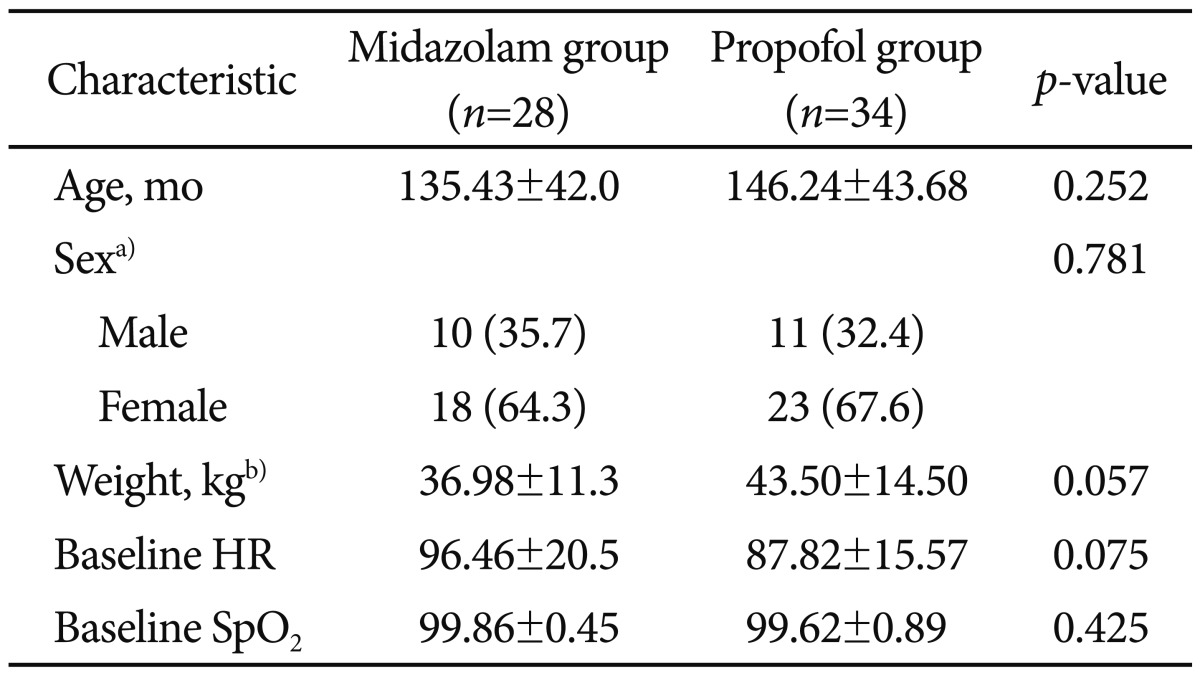
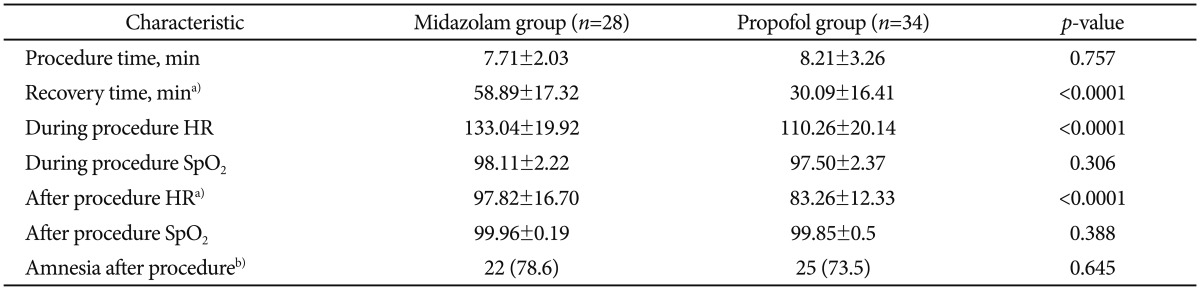
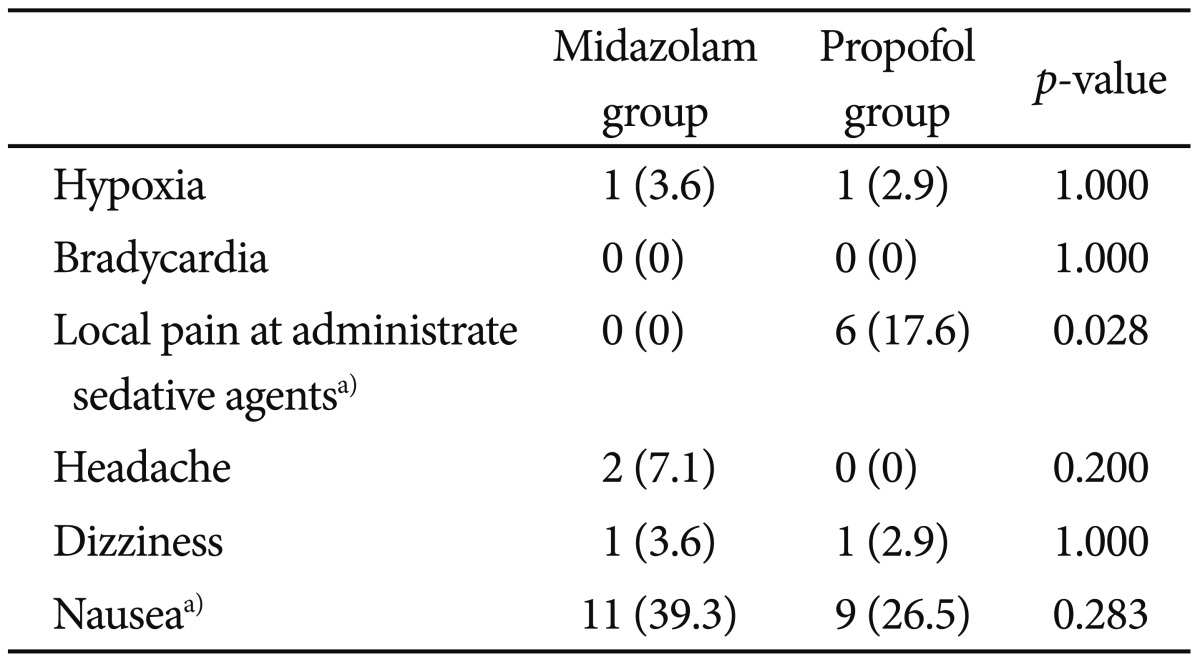
There were no statistically significant differences between the two groups in age, weight, sex, and the length of endoscopic procedure. The recovery time from sedation was markedly shorter in propofol group (30±16.41 minutes) compared with midazolam group (58.89±17.32 minutes; p<0.0001). During and after the procedure the mean heart rate was increased in midazolam group (133.04±19.92 and 97.82±16.7) compared with propofol group (110.26±20.14 and 83.26±12.33; p<0.0001). There was no localized pain during sedative administration in midazolam group, though six patients had localized pain during administration of propofol (p<0.028). There was no serious major complication associated with any of the 62 procedures.
Conclusions
Intravenous administered propofol provides faster recovery time and similarly safe sedation compared with midazolam in pediatric patients undergoing upper gastrointestinal endoscopy.
INTRODUCTION
Endoscopic procedures for diagnostic and therapeutic purposes in children are increasing. However, the method of sedation during the procedure remains controversial in children. These procedures often are invasive and result in anxiety and discomfort for the children and their parents. In addition, when a patient is unable to cooperate, the duration and potential complications of a procedure may increase.1 Conscious sedation has been accepted as the primary sedation in children during endoscopic procedures.2 To decrease the pain and anxiety associated with endoscopic procedure in children, optimized sedative agents are needed. A variety of agents are available for the sedation of children, but no ideal agent exists that allows rapid onset of action and recovery with minimal side effects.
Being a rapid acting sedative agent with minimal recovery time, propofol is a very effective sedative in children who are undergoing gastrointestinal (GI) endoscopy.3,4 It also has antiemetic and amnestic effect. However, there are well known adverse effects, in particular the dose-dependent potential to induce general anaesthesia or hemodynamic and respiratory depression, and the lack of a pharmacologic antagonist.5 For these reasons, the administration of propofol had been restricted primarily to anesthesiologists and nurse anesthetists trained in emergency airway management. There are several papers on the administration of propofol by nonanesthesiologists for gastroscopies in adults, and there have been increasing reports on the administration of propofol by trained nurses and pediatricians in defined settings.6-8
The standard sedation practice at our unit is intravenous (IV) midazolam and propofol for the majority of upper GI endoscopy. We report our experience on the use of propofol compare to midazolam in children undergoing diagnostic upper GI endoscopy.
MATERIALS AND METHODS
This was a retrospective study on children who underwent diagnostic upper GI endoscopy with propofol or midazaolam sedation at Samsung Changwon Hospital between August 1, 2010 and July 31, 2011. This retrospective study was approved by the Institutional Review Board of Samsung Changwon Hospital. Patients who meet any of the following criteria were included in this study: 1) children who are 4 to 17 years of age and 2) children who use sedative agents such as midazolam or propofol. Following these inclusion criteria, two investigators collected the data.
In our unit, endoscopist-directed use of propofol began in March 2010. All research published about endoscopy and the use of propofol was reviewed. All procedures were initially performed in cooperation with an anesthesiologist. All sedation and procedures were performed following the standardized Samsung Changwon Hospital protocol.
The pediatric endoscopist and nurses were certified in basic and advanced cardiac life support. All endoscopies were performed by a board-certified pediatric gastroenterologist and two endoscopy assistants. Medications were used for sedation at the discretion of the endoscopist.
All patients who underwent IV sedation were monitored continuously for heart rate (HR) and O2 saturation, with a sound alarm set at 90%. Supplemental oxygen was not administered routinely unless the SpO2 declined to less than 90% for a minimum of 10 seconds. The endoscopy assistants monitored chest movement, respiratory effort and respiratory rate. We could not check blood pressure because the patients were uncooperative, with crying and irritability during endoscopy.
There was no premedication prior to the endoscopy except lidocaine spray. The patients were given a spray of lidocaine 10% to the pharynx to reduce pain and discomfort during the procedure. They fasted for at least 8 hours. All patients had an IV line in place for the duration of endoscopic procedure and recovery.
Definitions of sedation levels, as developed and adopted by the American Society of Anesthesiologists, are given in Table 1.9,10 The pediatric endoscopist started the procedure once a patient was induced into moderate to deep sedation.
Following the standard protocol of our unit, midazolam (bolus dose 0.2 mg/kg, maximum total dose 0.3 mg/kg) or propofol was administered as bolus dosing by a trained endoscopy nurse under the pediatric endoscopist's supervision. Propofol was injected slowly, throughout at least 30 seconds, with an induction dose of 2 mg/kg in children up to 8 years of age and 1 to 2 mg/kg in older children. Repeated smaller dosages of 0.5 to 1 mg/kg were given to maintain sedation based on the patient's response. The usual interval between boluses was 1 minute. Two nurses helped the procedures: one to administer sedatives and monitor the patient and another to assist with the procedure.
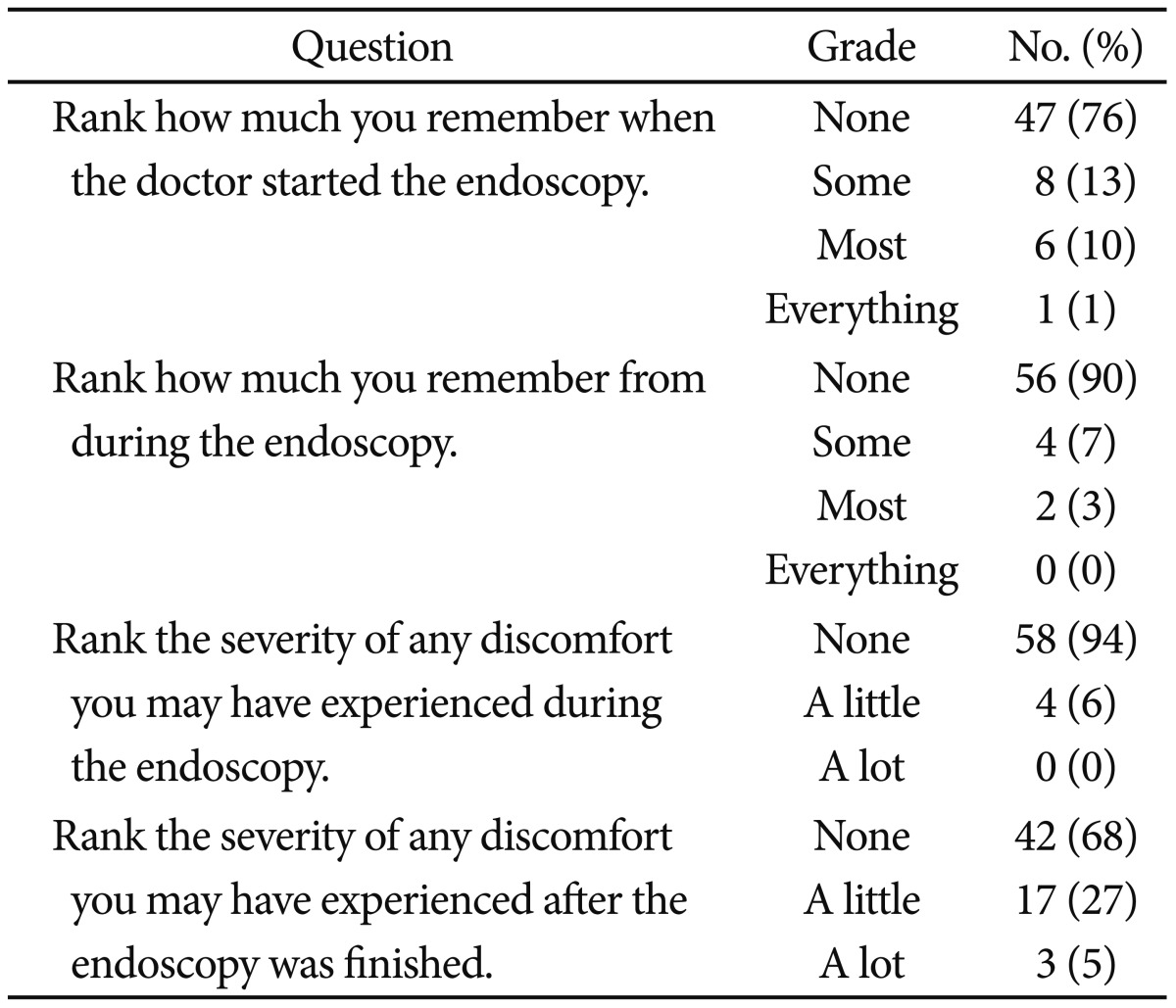
We used a postsedation questionnaire to assess the sedation efficacy since August 2010. The questionnaire referred to the previous study, and was designed to evaluate the memory and/or pain experienced children during the endoscopy.11 The questionnaire included the following questions:
1) Rank how much you remember when the doctor started the endoscopy.
2) Rank how much you remember during the endoscopy.
3) Rank the severity of any pain you may have experienced during the endoscopy.
4) Rank the severity of any pain you may have experienced after the procedure was completed.
Ranking was graded as none, some, most, everything. A recovery room nurse asked children about the questions in the questionnaire. At the time of the interview, patients completely alert and ready to be discharged. The questionnaire was completed by 62 patients.
The following data were reviewed: age, gender, weight, duration of procedure, recovery time, sedative agents, HR, and SpO2 at baseline, during the procedure and after the procedure, and side effects. Duration of procedure was calculated as the time from the start of the procedure (insertion of the endoscope orally) to the end of the procedure (time of withdrawal of the endoscope). Recovery time was defined as the time from administration of the sedatives to the time when patients were awake and verbal.
All statistical analyses were performed with SPSS version 18 (IBM Co., Armonk, NY, USA). Unpaired Student t-test or Mann-Whitney U test was used as appropriate to compare continuous data. Categorical variables were tested using corrected chi-square or Fisher exact tests for univariate comparisons, as appropriate. Null hypotheses of no difference were rejected if p-values were less than 0.05.
RESULTS
During the study period, 91 children (32 boys and 47 girls) underwent diagnostic upper endoscopic procedure. According to the inclusion criteria, we reviewed the charts of 62 patients (21 boys and 41 girls). The mean age of patients was 135±42 months in the midazolam and 146±43 months on the propofol group. There were 11 boys and 23 girls in the propofol group and 10 boys and 18 girls in the midazolam group. The average duration of the endoscopic procedures was 7.71±2 minutes in the midazolam group and 8.21±3.26 minutes in the propofol group. Demographic data of both groups are shown in Tables 2, 3. There were no statistically significant differences between the two groups in terms of age, weight, sex, and length of the endoscopic procedure.
The mean time to wake-up was 28.5 minutes in the propofol gourp and 55 minutes in the midazolam group. The recovery time from sedation was markedly shorter in the propofol group (30±16.41 minutes) compared with the midazolam group (58.89±17.32 minutes; p<0.0001). Mean HR during and after the procedure were increased in the midazolam group (133.04±19.92 and 97.82±16.7, respectively) compared with the propofol group (110.26±20.14 and 83.26±12.33, respectively; p<0.0001). The mean dose of propofol was 2.32 mg/kg and the mean dose of midazolam was 0.15 mg/kg for endoscopy.
We reviewed postsedation questionnaires (Table 4). Over 95% had little or no pain during the endoscopy and over 90% had some or no memory during the procedure. In question 3 and 4, 24 patients had a little or a lot of discomfort during and after the endoscopy. The nurse asked the patients if they had any discomfort and, if so, to record what kind of discomfort it was. Among the 24 patients, two patients had headache, two patents had dizziness, and 20 patients had nausea. Side effects of sedation are presented in the Table 5.
There was no serious major complication associated with any of the 62 procedures. Two patients developed respiratory adverse events with oxygen desaturation (SpO2 was 85% to 90%) after administering the IV sedative drugs and required the administration of supplemental oxygen. One of the two patients was in the propofol group and the other was in the midazolam group. In all cases, the SpO2 was recovered above 95% by using the chin thrust maneuver and supplemental oxygen. But there was no cardiovascular adverse event. There was no localized pain during sedative administration in midazolam group, though six patients had localized pain during the administration of propofol (p<0.028). Headache, dizziness, nausea, and memory loss after the procedure were not different between the two groups.
DISCUSSION
As endoscopic procedure for pediatric GI diseases increases, the need for deep sedation for children has also increased. But there is no ideal protocol for sedation in children during endoscopic procedures. Different approaches have been adopted in various hospitals, according to their experiences and technology.12 In many endoscopy units, midazolam is the IV sedative drug of choice. Midazolam is one of benzodiazepines, with 1 to 5 minutes of onset of action and peak effect within 3 to 5 minutes.13 Midazolam is used frequently because it has potent amnestic and some anxiolytic effects.14 However, midazolam often do not provide adequate patient comfort during endoscopic procedures. Lamireau et al.15 found that incomplete procedures and complete procedures under difficult conditions occurred more frequently in patients administered with IV midazolam with general anesthesia. They also found that HR and blood pressure increased significantly during endoscopy in the midazolam sedation group.15 Our study showed that HR increased significantly during and after the endoscopic procedure in patients sedated with IV midazolam compared with propofol.
The ideal sedative agent for endoscopic procedures should have a rapid onset of action, produce a level of sedation sufficient for patient comfort, and have a short duration of action.8 So there is an increasing interest in the use of propofol. In our unit, propofol was used according to previous study about nonanesthesiologist administration of propofol for GI endoscopy.6-8
Propofol is primarily a hypnotic compound, which provides amnesia. It has an extremely rapid onset of action, producing unconsciousness within 30 to 60 seconds, and a short half-life (1.3 to 4.3 minutes).16 A pharmacologic disadvantage of propofol is its relatively narrow therapeutic range. Unlike opioids and benzodiazepines, an antagonist is not available to reverse the effects. Given its high potential to induce respiratory depression and cardiovascular instability, propofol has been routinely administered by anesthesiologist.13 However, debate continues as to whether propofol should be administered only by anesthesiologist, assistant endoscopists or trained nurses.16 Because of limited anesthesiology resources, propofol is being administered worldwide by nonanesthesiologists such as trained nurses or endoscopists for endoscopy in adult patients.17 Further strong evidence exists demonstrating that well trained nonanesthesiologists may provide safe and effective propofol sedation in children, including for endoscopic procedures.18 The present review suggests propofol-based procedural sedation is the most effective regimen for procedural sedation during endoscopy in children.10
Previous researches suggest that propofol (versus midazolam and meperidine) for sedation during endoscopy is safe, produces quicker induction of sedation, faster recovery, effective amnesia, faster return to baseline activities and is probably cost-effective.19 Our study shows that propofol was associated with faster recovery compared with the midazolam group. There was no difference in procedure time, incidence of amnesia or side effects after procedure.
Propofol has vasodilator properties and may cause reduced cardiac contractility and negative chronotropic effects.20 Propofol is associated with a relatively higher incidence of respiratory depression, apnea, and hypotension.21 But in our study, there were no serious complications, such as intubation or ambu bag assistance, during endoscopy and all procedures were performed successfully. Only two patients needed supplemental oxygen after the administration of IV sedative drugs. One of these two patients was in the propofol group and the other was in the midazolam group. It has been known that about 60% of patients experience pain on injection with standard propofol alone.22 In our study, six patients (17.6%) developed local pain during IV propofol administration, but the pain was generally mild in nature.
Our results indicate that intravenously administered propofol produces faster recovery time and safe sedation similar to midazolam in pediatric patients undergoing upper GI endoscopy. Similar findings have been reported in studies evaluating sedation in children when propofol is administered.3,6,10,12,18 While this study is the first to compare the efficacy and safety of IV administered midazolam and propofol for sedation in children during endoscopic procedures in Korea, our study has limitations of a retrospective single-center study with small sample size. Thus, a patient selection bias cannot be excluded and data on induction time of sedation and patient's satisfaction with sedation are lacking. Further prospective data and large clinical trials are required to support this finding and assess the frequency of serious adverse events.
Acknowledgments
This work was supported by the Hyosuk Research Fund from Samsung Changwon Hospital, Sungkyunkwan University School of Medicine.
Notes
The authors have no financial conflicts of interest.