AbstractBackground/AimsThe pathogenesis of bone loss in patients with inflammatory bowel disease (IBD) is complex, multifactorial, and only partly understood. We aimed to examine the extent and risk factors of bone mass reduction and to analyze the impact of early onset of a disease before attaining peak bone mass in IBD patients.
MethodsWe compared the risk factors for osteoporosis and BMD at the lumbar spine and the hip bone in IBD patients.
ResultsA total of 44 patients with IBD were enrolled. Twenty-one and 23 patients were diagnosed as IBD before and after the age of 30 and designated as group A and group B, respectively. Group A had significant bone mass reduction at the lumbar spine than group B (BMD, 1.01┬▒0.10 vs. 1.14┬▒0.17, p<0.01; T-score, -1.22┬▒0.84 vs. -0.08┬▒1.39, p<0.01; Z-score, -1.11┬▒0.81 vs. -0.03┬▒1.32, p<0.01, respectively). Multivariate analysis showed that patients diagnosed as IBD before the age of 30 had possible risk factor of bone mass reduction (hazard ratio, 3.96; p=0.06).
INTRODUCTIONPatients with inflammatory bowel disease (IBD) have an increased risk for loss of bone mass. Osteoporosis is characterized by low bone mineral density (BMD) and deteriorated microarchitecture of the bone tissue. The pathophysiology of IBD-related osteoporosis is presumably multifactorial and not fully understood;1,2 however, risk factors such as steroid treatment, systemic effects of chronic inflammation, calcium and vitamin D deficiencies, and malnutrition are known to be involved.3 Recent data have indicated that osteopenia associated with Crohn's disease (CD) correlates with the basic pathology of CD rather than malabsorption or complications of steroid treatment.4,5
Low BMD in a premenopausal woman may result from attaining peak bone mass that is below average due to genetic predisposition, illnesses or medications that negatively impact bone density accrual. Population-based, cross-sectional studies suggest that women attain peak bone mass at the proximal femur in their 20s and at the spine and forearm around the age of 30.6,7
The aims of this study were to examine the extent and risk factors of bone mass reduction and to analyze the impact of early onset of a disease before attaining peak bone mass in IBD patients.
MATERIALS AND METHODSPatientsFrom September 2010 through November 2010, we enrolled patients with IBD, aged between 18 and 70 years, who visited Soonchunhyang University Bucheon Hospital. The diagnosis of IBD had been confirmed on clinical, endoscopic, radiologic, and histologic examinations. Patients with colectomy, menopausal state, diabetes mellitus, thyroid disease, parathyroid disease, chronic liver disease, chronic kidney disease (CrCl <50 mm/min), sexual dysfunction, history of femur fracture, metabolic bone disease (including hypercalcemia or hypocalcemia), or a history of vitamin D supplement, sex hormone, or previous bisphosphonate treatment were excluded. Forty-nine patients with IBD participated; five were excluded, and a total of 44 patients were enrolled in this study. The main demographic data, major points of the clinical history (sex, age, age at diagnosis, disease activity, hospitalization, localization, duration of the disease, surgery, drug use, and treatment), and risk factors for osteoporosis (body mass index, menopausal state, smoking, previous bone fracture, and calcium intake) were assessed by interviews and from medical records. Clinical and laboratory data were compared between two groups that were divided by the age of 30, when the bone mass is peaked. For the comparison of BMD, age- and sex-matched 110 controls without IBD were enrolled.
BMDThe measurement sites were the lumbar vertebrae from L1 to L4 in the spine and the hip (femoral neck and total hip). The BMD measurements were made by one trained person using dual-energy X-ray absorptiometry equipment (PRODIGY; GE Lunar Co., Milwaukee, WI, USA). Bone density was expressed as BMD (g/cm2), Z-score (the number of standard deviations [SD] from the mean of gender- and age-matched controls), and T-score (the number of SD from the mean of healthy young adults who have attained peak bone mass). Dichotomizing the Z-score, a cutoff value of Ōēż-1 SD was defined as reduced bone mass and Ōēż-2 SD as severely reduced bone mass. X-ray of the lumbar spine was performed in all patients, and the images were evaluated by a trained radiologist.
BiochemistryCalcium, phosphorus, intact parathyroid hormone (i-PTH), 25(OH)-vitamin D3, and biochemical markers (osteocalcin [OC], deoxypyridinoline) were measured to assess changes in bone metabolism; these examinations were performed on the same occasion as the BMD test after 12 hours of fasting. Also, a urine deoxypyridinoline test was performed using the first voided morning urine specimen. Calcium and phosphorus were measured by standard Technicon AutoAnalyzer methods. Serum i-PTH and OC were measured by an electrochemiluminescent immunoassay, serum 25(OH)-vitamin D3 was measured using a chemiluminescent immunoassay, and urine deoxypyridinoline was measured via a radioimmunoassay. We defined 25(OH)-vitamin D3 levels <10 ng/mL as representing vitamin D deficiency, those between 10 and 30 ng/mL as vitamin D insufficiency, and those >30 ng/mL as vitamin D sufficiency. The upper limit of OC was 31.9 ng/mL in premenopausal females and 37.0 ng/mL in males. The normal urine deoxypyridinoline concentration in the serum was 2.5 to 6.5 nM/mmol.
Dietary calcium intakeDietary calcium intake was assessed by an experienced dietician using the recall method.
Statistical analysisStatistical analysis was performed using SPSS software version 14.0 (SPSS Inc., Chicago, IL, USA). Continuous variables were compared using Student's t-test and categorical variables were compared using chi-square or Fisher's exact test between the two groups. To predict independent risk factors of BMD reduction, multivariate analysis by logistic regression was used. For all tests, a p<0.05 was considered statistically significant.
RESULTSPatient characteristicsForty-four patients with IBD were enrolled in this study. These patients were divided into two groups according to their age at diagnosis. Twenty-one and 23 patients were diagnosed as IBD before and after the age of 30 and designated as group A and B, respectively. Group A had significantly longer hospitalization time than group B (27.5┬▒36.7 days in group A, 6.8┬▒8.8 days in group B; p=0.02). There was no significant difference in calcium intake between the two groups (555.6┬▒308.1 mg in group A, 580.4┬▒232.0 mg in group B; p=0.18). Group A had significantly higher level of 25(OH)-vitamin D3 than group B (15.2┬▒6.4 ng/mL in group A, 12.0┬▒4.7 ng/mL in group B; p=0.03). Eighteen (40.9%) of 44 patients had vitamin D deficiency and the remaining 26 patients (59.1%) had vitamin D insufficiency. No significant differences existed between group A and group B in disease duration, total duration of steroid, cumulative steroid dose, C-reactive protein (CRP), smoking, and alcohol, serum calcium, phosphorus, and i-PTH level (Table 1). No patients had any bone fractures.
BMDs between patients with IBD and age- and sex-matched controls without IBDIn comparison with age- and sex-matched controls, group A showed statistically significant difference in BMD, T-score, Z-score at spine L1-4 (1.01┬▒0.10 vs. 1.12┬▒0.14, p=0.01; -1.22┬▒0.84 vs. -0.22┬▒1.19, p<0.01; -1.11┬▒0.81 vs. -0.42┬▒1.12, p=0.02, respectively). However there was no significant difference in bone mass reduction at other bones. In comparison with ageand sex-matched controls, group B showed no significant difference in BMD, T-score, Z-score at any site measured (Table 2).
Risk factors of bone mass reduction in patients with IBDNineteen (43.2%) of patients with IBD had reduced bone mass (Z-score Ōēż-1). Fifteen patients had moderately reduced bone mass (-2 SD<Z-scoreŌēż-1 SD), and four patients had severely reduced bone mass (Z-score Ōēż-2 SD). Two of the patients with reduced bone mass were diagnosed with osteoporosis, as their T-scores were below -2.5 SD. No significant differences in clinical and biochemical bone markers were observed between patients with reduced bone mass (Z-score Ōēż-1 SD) and those with normal bone mass (Table 3). No statistically significant difference in bone density was found between patients with UC and those with CD at any site measured (Table 4). Of the 44 patients, 35 patients were taking or had taken corticosteroids and nine patients had never taken steroids. BMD, T-score, and Z-score at the spine were significantly higher in the patients who never used steroids (1.22┬▒0.16 vs. 1.05┬▒0.13, p<0.01; 0.48┬▒1.34 vs. -0.91┬▒1.12, p<0.01; 0.48┬▒1.33 vs. -0.81┬▒1.12, p<0.02) (Table 5). Table 6 shows significant differences in BMD, T-score, Z-score at the spine between group A and B (1.01┬▒0.10 vs. 1.14┬▒0.17, p<0.01; -1.22┬▒0.84 vs. -0.08┬▒1.39, p<0.01; -1.11┬▒0.81 vs. -0.03┬▒1.32, p<0.01). There was no significant difference in bone mass reduction at femur neck and hip bones, however. Multivariate analysis was carried out to analyze risk factors of reduced BMD, including age at diagnosis, inflammation, CRP, dose of steroids, and calcium and vitamin D intake. The result showed the age at diagnosis was a possible risk factor (hazard ratio, 3.96; p=0.06), although there was no statistical significance (Table 7).
DISCUSSIONThe incidence of IBD has been rising not only in Western countries, but also in Asia.8 IBD is a chronic relapsing and remitting inflammatory disorder of the bowel. Osteoporosis is an established complication in patients with IBD, and the clinical consequence of osteoporosis is increased risk of fracture.
Most of the early studies show cumulative epidemiologic data.9-11 Osteoporosis and low bone mass has been found in as many as 30.6% of 75 unselected IBD cases.9 Also, the rate of osteoporosis defined by a T-score of below -2.5 was 15% in a very similar IBD population.12 Further uncontrolled studies have reported a prevalence of osteoporosis in CD patients as low as 12%,13,14 but that of osteoporosis was estimated to be as high as 18% to 42% in unselected cohorts.15,16 Also, similar to other early studies on prevalence, our study showed that 19 (43%) of 44 patients have low bone mass. Two (10.5%) of the patients with reduced bone mass were diagnosed with osteoporosis, as their T-scores were below -2.5 SD. Because most of our patients were diagnosed within the last 10 years, the period of illness was shorter than the Western patients.
The pathogenesis of osteoporosis in patients with IBD is unclear and most likely multifactorial. Several factors including age, steroids use, chronic inflammation, malnutrition, vitamin D, and calcium deficiencies, and immobilization have important effects.17 Chronic inflammatory state leads to increased intestinal cytokines (e.g., interleukin-1 and -6, tumor necrosis factor) that stimulate bone resorption.2,4,17 Steroids cause bone loss by inhibiting bone formation, increasing bone resorption, decreasing intestinal absorption of calcium, and increasing renal excretion of calcium. In the past, steroid use was considered a major cause of osteoporosis in patients with IBD.18 More recently, the inflammatory process itself has been suggested to play a pivotal role in IBD-associated bone loss.19-21 However, it is difficult to distinguish the adverse skeletal effect of steroids from those of disease-related inflammatory activity because the two factors are often linked.17 In our study, three of nine patients who were not administrated steroids had decreased BMD (Z-score Ōēż-1 SD). The result of this study revealed that patients with current or past use of steroids had significantly lower BMD at the spine than patients who never used steroids. Although cumulative steroid dose was not a significant risk factor in the multivariate analysis, we think that steroid use may be one of causes of BMD reduction.
Vitamin D deficiency is not only a consequence but also a cause of inflammatory processes leading to bone loss. Studies investigating vitamin D status in patients with IBD have had conflicting results.16,22-26 In this study, all of 44 patients had vitamin D deficiency or insufficiency status. The levels of 25 (OH)-vitamin D3 were 15.2┬▒6.4 ng/mL in group A and 12.0┬▒4.7 ng/mL in group B (p=0.03). However, vitamin D deficiency was not a significant risk factor of BMD reduction in patients with IBD in the multivariate analysis.
Peak bone mass can be defined as the maximal BMD that has been accrued during growth and development. The precise age at which peak BMD is acquired is still unknown and may be site-dependent. Maximal bone density is generally accepted to be present during the third to fourth decade of life.27 Because peak bone mass is usually achieved during the third to fourth decade of life, retardation of growth and skeletal maturation are common in children with IBD.28-31 Therefore, we divided enrolled patients into two groups by the age of 30 when the peak bone mass is achieved (group A patients who were diagnosed as IBD before the age of 30; group B patients who were diagnosed as IBD after the age of 30). In our study, group A had lower BMD than group B, although patients had IBD during similar period. The results of our study also showed that BMD reduction was observed mainly in the lumbar spine. However, there was no significant difference in BMD reduction between UC and CD.
This study had some limitations: 1) The sample size of patients with IBD was too small to reach a sufficient conclusion and to confirm the present results. 2) There was no sufficient evaluation for various risk factors associated with bone mass reduction such as calcium and vitamin D intake, malnutrition, smoking, alcohol use, physical activity, and inflammatory cytokines.
In conclusion, bone mass reduction in patients with IBD occurred mainly in the lumbar spine. Although we could not find a significant difference, reduction of bone mass was more severe in patients who were diagnosed as IBD before the age of 30 than those who were diagnosed as IBD after the age of 30. Therefore, bone mass reduction should be monitored closely in patients who were diagnosed as IBD before the age 30, and other rescue medications that can substitute steroids are needed for these patients.
References1. Bernstein CN, Leslie WD. Review article: osteoporosis and inflammatory bowel disease. Aliment Pharmacol Ther 2004;19:941ŌĆō952. 15113361.
2. Ali T, Lam D, Bronze MS, Humphrey MB. Osteoporosis in inflammatory bowel disease. Am J Med 2009;122:599ŌĆō604. 19559158.
3. Vestergaard P. Bone loss associated with gastrointestinal disease: prevalence and pathogenesis. Eur J Gastroenterol Hepatol 2003;15:851ŌĆō856. 12867793.
4. Moschen AR, Kaser A, Enrich B, et al. The RANKL/OPG system is activated in inflammatory bowel disease and relates to the state of bone loss. Gut 2005;54:479ŌĆō487. 15753532.
5. Miheller P, Muzes G, Zagoni T, Toth M, Racz K, Tulassay Z. Infliximab therapy improves the bone metabolism in fistulizing Crohn's disease. Dig Dis 2006;24:201ŌĆō206. 16699279.
6. Tenenhouse A, Joseph L, Kreiger N, et al. Estimation of the prevalence of low bone density in Canadian women and men using a population-specific DXA reference standard: the Canadian Multicentre Osteoporosis Study (CaMos). Osteoporos Int 2000;11:897ŌĆō904. 11199195.
7. Lofman O, Larsson L, Toss G. Bone mineral density in diagnosis of osteoporosis: reference population, definition of peak bone mass, and measured site determine prevalence. J Clin Densitom 2000;3:177ŌĆō186. 10871911.
8. Yang SK, Yun S, Kim JH, et al. Epidemiology of inflammatory bowel disease in the Songpa-Kangdong district, Seoul, Korea, 1986-2005: a KASID study. Inflamm Bowel Dis 2008;14:542ŌĆō549. 17941073.
9. Compston JE, Judd D, Crawley EO, et al. Osteoporosis in patients with inflammatory bowel disease. Gut 1987;28:410ŌĆō415. 3583068.
10. Szathm├Īri M, Pr├│nai L, Tulassay Z. Altered bone metabolism in inflammatory bowel disease. Am J Gastroenterol 1998;93:848ŌĆō849. 9625151.
11. Pigot F, Roux C, Chaussade S, et al. Low bone mineral density in patients with inflammatory bowel disease. Dig Dis Sci 1992;37:1396ŌĆō1403. 1505291.
12. Schulte C, Dignass AU, Mann K, Goebell H. Reduced bone mineral density and unbalanced bone metabolism in patients with inflammatory bowel disease. Inflamm Bowel Dis 1998;4:268ŌĆō275. 9836078.
13. Schoon EJ, van Nunen AB, Wouters RS, Stockbr├╝gger RW, Russel MG. Osteopenia and osteoporosis in Crohn's disease: prevalence in a Dutch population-based cohort. Scand J Gastroenterol Suppl 2000;(232):43ŌĆō47. 11232491.
14. Robinson RJ, al-Azzawi F, Iqbal SJ, et al. Osteoporosis and determinants of bone density in patients with Crohn's disease. Dig Dis Sci 1998;43:2500ŌĆō2506. 9824142.
15. Bernstein CN, Seeger LL, Sayre JW, Anton PA, Artinian L, Shanahan F. Decreased bone density in inflammatory bowel disease is related to corticosteroid use and not disease diagnosis. J Bone Miner Res 1995;10:250ŌĆō256. 7754804.
16. Bjarnason I, Macpherson A, Mackintosh C, Buxton-Thomas M, Forgacs I, Moniz C. Reduced bone density in patients with inflammatory bowel disease. Gut 1997;40:228ŌĆō233. 9071937.
17. Bernstein CN, Leslie WD, Leboff MS. AGA technical review on osteoporosis in gastrointestinal diseases. Gastroenterology 2003;124:795ŌĆō841. 12612917.
18. Jahnsen J, Falch JA, Aadland E, Mowinckel P. Bone mineral density is reduced in patients with Crohn's disease but not in patients with ulcerative colitis: a population based study. Gut 1997;40:313ŌĆō319. 9135518.
19. Walsh MC, Kim N, Kadono Y, et al. Osteoimmunology: interplay between the immune system and bone metabolism. Annu Rev Immunol 2006;24:33ŌĆō63. 16551243.
20. Henderson S, Hoffman N, Prince R. A double-blind placebo-controlled study of the effects of the bisphosphonate risedronate on bone mass in patients with inflammatory bowel disease. Am J Gastroenterol 2006;101:119ŌĆō123. 16405543.
21. Oostlander AE, Bravenboer N, Sohl E, et al. Histomorphometric analysis reveals reduced bone mass and bone formation in patients with quiescent Crohn's disease. Gastroenterology 2011;140:116ŌĆō123. 20854819.
22. Ghosh S, Cowen S, Hannan WJ, Ferguson A. Low bone mineral density in Crohn's disease, but not in ulcerative colitis, at diagnosis. Gastroenterology 1994;107:1031ŌĆō1039. 7926456.
23. Abitbol V, Roux C, Chaussade S, et al. Metabolic bone assessment in patients with inflammatory bowel disease. Gastroenterology 1995;108:417ŌĆō422. 7835582.
24. Sonnenberg A, Ehms H, Sonnenberg GE, Strohmeyer G. 25-hydroxycholecalciferol serum levels in patients with Crohn's disease. Acta Hepatogastroenterol (Stuttg) 1977;24:293ŌĆō295. 906778.
25. Driscoll RH Jr, Meredith SC, Sitrin M, Rosenberg IH. Vitamin D deficiency and bone disease in patients with Crohn's disease. Gastroenterology 1982;83:1252ŌĆō1258. 6982188.
26. Compston JE, Creamer B. Plasma levels and intestinal absorption of 25-hydroxyvitamin D in patients with small bowel resection. Gut 1977;18:171ŌĆō175. 856674.
27. Soyka LA, Fairfield WP, Klibanski A. Clinical review 117: hormonal determinants and disorders of peak bone mass in children. J Clin Endocrinol Metab 2000;85:3951ŌĆō3963. 11095413.
28. Kirschner BS, Voinchet O, Rosenberg IH. Growth retardation in inflammatory bowel disease. Gastroenterology 1978;75:504ŌĆō511. 680509.
29. Griffiths AM, Nguyen P, Smith C, MacMillan JH, Sherman PM. Growth and clinical course of children with Crohn's disease. Gut 1993;34:939ŌĆō943. 8344582.
Table┬Ā1Baseline Characteristics of Enrolled Patients  Values are presented as mean┬▒SD or number (%). M, male; F, female; UC, ulcerative colitis; CD, Crohn's disease; BMI, body mass index; i-PTH, intact parathyroid hormone; CRP, C-reactive protein; RDW, red blood cell distribution width. a)Group A patients who diagnosed as inflammatory bowel disease (IBD) before age of 30; b)Group B patients who diagnosed as IBD after age of 30. Table┬Ā2Comparison of Bone Density Status between Patients with IBD and Age- and Sex-Matched Controls without IBD 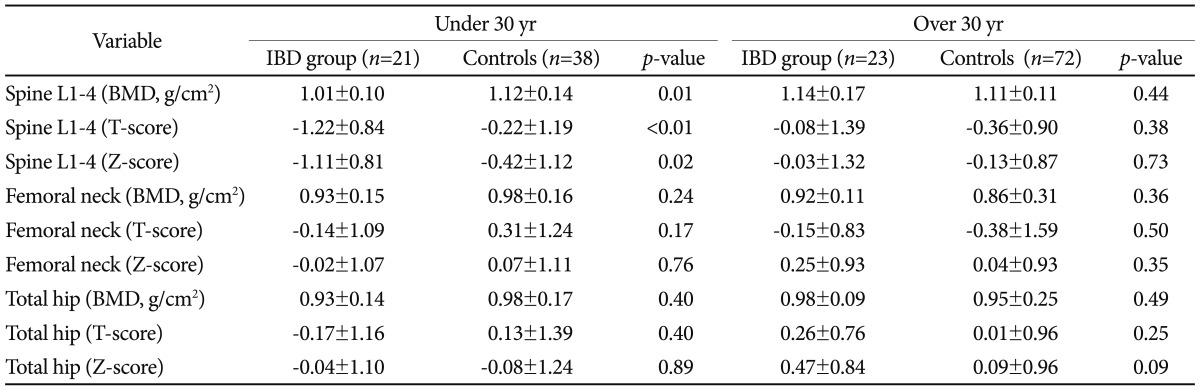
Table┬Ā3Comparison of Clinical and Biochemical Parameters between Inflammatory Bowel Disease Patients with Normal and Reduced BMD 
Table┬Ā5Comparison of Bone Density Status between Patients Who Used Steroids and Those Who Never Used Steroids 
|
|
||||||||||||||||||||||||||||||||||||||||||||
