A Case of Malignant Granular Cell Tumor in the Sigmoid Colon
Article information
Abstract
Granular cell tumor (GCT) is an uncommon, usually benign neoplasm; however, a malignant potential has been described. Malignant GCT is an extremely rare neoplasm showing rapid growth and invasion into adjacent muscles, lymph nodes, or vessels, or even distant metastasis. We recently experienced a case of a histologically benign or atypical but clinically malignant GCT, with invasion of the lymph nodes and vessels in the sigmoid colon, diagnosed by segmental colon resection with lymph node dissection. We also performed a review of relevant medical literature.
INTRODUCTION
Granular cell tumor (GCT) is a benign tumor believed to originate from neural cells; it may occur at many sites, although it occasionally affects the oral cavity, skin, soft tissue, breast, and female genital organs.1,2 The gastrointestinal tract is an uncommon location of GCTs, and the most common site is the esophagus followed by the large intestine and stomach.1,3 Most of the colorectal GCTs were located in the proximal colon, cecum, appendix, and rectum, and they usually appeared as a solitary growth and followed a benign course.4 Although GCTs are usually benign, malignant transformation or de novo primary malignant tumors have been described, particularly for large lesions.5,6
To date, only a few cases of benign GCT in the sigmoid colon have been reported, and to our knowledge, there is only one case report in the English literature of malignant GCT in the colon incidentally detected by positron emission tomography/computed tomography.3,7,8,9 Here, we report a second case, involving a 43-year-old woman with histologically benign or atypical but clinically malignant GCT with invasion of the lymph nodes and vessels in the sigmoid colon, and treated by segmental colon resection with lymph node dissection.
CASE REPORT
A 43-year-old female patient visited SAM Medical Center for further evaluation of a submucosal tumor that was seen on prior colonoscopy in another clinic. She was asymptomatic, and a physical examination showed unremarkable findings. The laboratory results showed a white blood cell count of 4,100/mm2, hemoglobin of 7.7 g/dL, platelet count of 340,000/mm2, albumin of 4.4 g/dL, aspartate aminotransferase of 13 IU/L, and alanine aminotransferase of 7I IU/L. Colonoscopy showed a yellowish, round submucosal tumor with intact mucosa, approximately 1 cm in diameter in the sigmoid colon (Fig. 1). Endoscopic ultrasonography (EUS) revealed an approximately 1×1 cm hypoechoic heterogeneous mass with a smooth margin originating from the third echo layer (Fig. 2). Because the diagnosis was benign GCT or carcinoid tumor, and the tumor was relatively small, we decided to follow the patient after 1 year, after she was given sufficient explanation about the malignant potential of the lesion. At the follow-up colonoscopy 1 year later, the size of the tumor increased to approximately 3 cm. We performed segmental colon resection with lymph node dissection, and the pathologic gross finding showed the tumor to be well circumscribed and located at the submucosal and muscular layers with central infiltration of the serosa. Histologic examination revealed proliferation of large polygonal cells with abundant eosinophilic granular cytoplasm, which was positive for S-100 and invasion of local lymph nodes and vessels (Fig. 3). Two of the histologically malignant criteria of GCT-presence of vesicular nuclei with large nucleoli and pleomorphism-were met, and the tumor was diagnosed as a histologically benign or atypical but clinically malignant GCT occurring in the sigmoid colon.
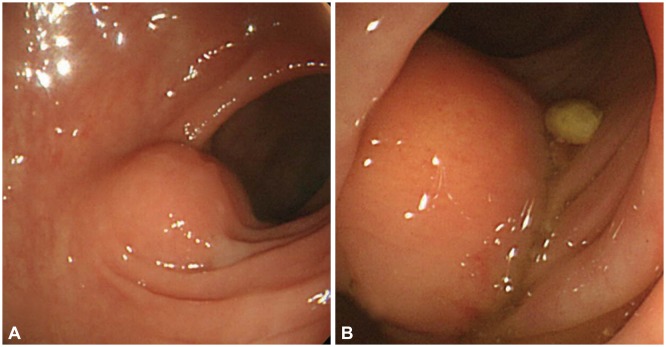
Colonoscopic findings. (A) An approximately 1-cm yellowish round submucosal tumor was observed in the sigmoid colon. (B) Follow-up colonoscopy 1 year later showed that the submucosal mass increased in size to approximately 3 cm.
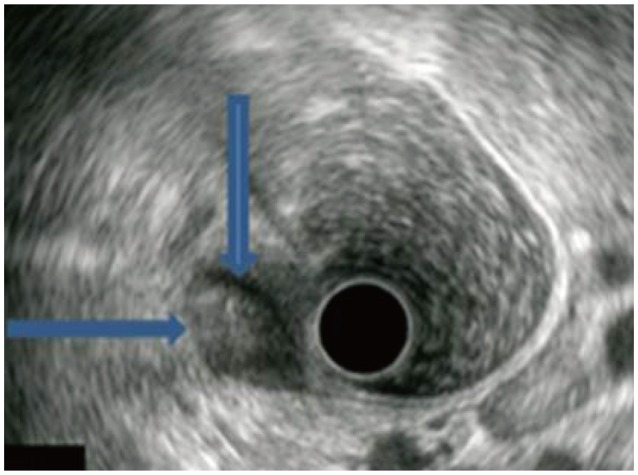
Endoscopic ultrasound findings. An approximately 1×1-cm heterogeneous hypoechoic tumor with well-demarcated border (arrows) was found on the submucosal layer.
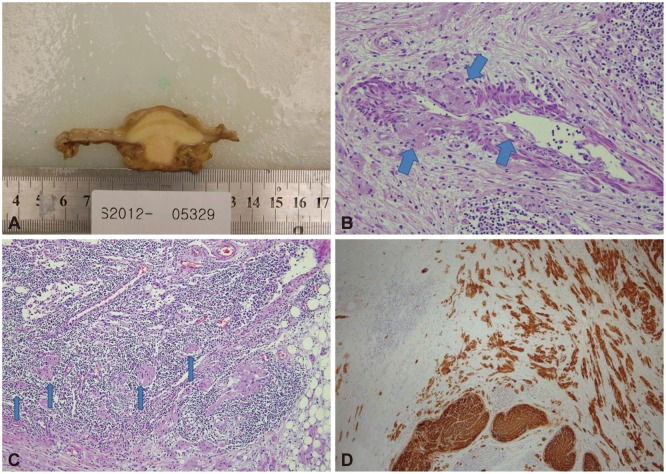
Pathologic findings. (A) Gross findings after segmental colon resection revealed that the mass was well circumscribed and located at the submucosal and muscular layers with central infiltration of the serosa. Histologic examination showed (B) proliferation of large polygonal cells with abundant eosinophilic granular cytoplasm and vascular invasion of tumor cells (thick arrows) (H&E stain, ×200), (C) perinodal lymphatic invasion of tumor cells (thin arrows) (H&E stain, ×100), and (D) immunohistochemical positivity for S-100 protein (S-100 protein, ×40).
DISCUSSION
GCT, which was first described occurring in the tongue by Abrikossoff in 1926, is a rare, benign tumor that can occur anywhere in the body; however, only approximately 8% of GCTs are found in the gastrointestinal tract.10 In endoscopic findings, gastrointestinal GCTs are typically yellow-white, sessile, small (<20 mm), and covered by a normal-appearing mucosa.3 On endosonography, GCTs are usually hypoechoic, homogeneous lesions in the submucosa, and these features are helpful for determining the depth of invasion and planning the surveillance and therapeutic strategy.3,7 In our patient, EUS showed a hypoechoic, heterogeneous submucosal tumor originating from the third echo layer, and the lesion was initially considered as a benign GCT or carcinoid tumor.
The origin of GCTs has not yet been definitely determined, and the recent use of immunohistochemical stains, including S-100 protein or neuron-specific enolase (NSE), suggests its origin to be the Schwann cells as well as the possibility of a neuroendocrine origin.2 Histologically, GCTs are composed of large polygonal cells containing numerous eosinophilic granules and small, uniform nuclei and neural markers, including S-100 protein or NSE.7
Although a GCT usually appears as a solitary small nodular growth and follows a benign course, a malignant potential has been described in a few cases, particularly for large lesions >4 cm in diameter.7 Comparisons between malignant transformation and de novo primary malignant tumor are based on primary histologic findings and the clinical course of the patient, especially in recurrent cases during the follow-up period. Malignant GCT is usually difficult to diagnose because of its rarity, and it was first described by Ravich in 1945.11 These tumors have been classified into two categories: histologically and clinically malignant type, and histologically benign but clinically malignant type. Although metastasis, among the worse prognostic features including a larger tumor size, advanced patient age, and local tumor recurrence, remains the most important criteria for defining malignancy, not all malignant GCTs actually metastasize. Therefore, attempts have been made to identify specific malignant histologic features to predict malignant behavior. Histologically malignant features include spindling of tumor cells, the presence of vesicular nuclei with large nucleoli, increased mitotic rate, a high nuclearto-cytoplasmic ratio, pleomorphism, and necrosis, and malignant GCT is histologically diagnosed when three or more of those six criteria are fulfilled. Tumors that meet one or two criteria are histologically diagnosed as atypical, and those that exhibit only focal pleomorphism are diagnosed as benign.5 Because malignancy can be definitely proven only by clinical findings, especially metastasis, clinical features such as metastasis, large size, rapid growth, and invasion into adjacent tissue are reported to be more important criteria of malignancy than the histologic features of a tumor with histologically undetermined malignant potential.7,12 In our patient, two microscopic criteria-the presence of vesicular nuclei with large nucleoli and pleomorphism-were observed in the resected tumor, and the lesion was considered as histologically benign or atypical; however, the lesion manifested clinically malignant features such as rapid growth and invasion of lymph node and vessel. Therefore, this case can be classified as a histologically benign or atypical but clinically malignant type of GCT. Some similar cases of histologically benign or atypical GCT, which were diagnosed using the classic histological criteria, were reported previously as a malignant GCT owing to their clinical course.5,12
In addition, malignant transformation from a benign tumor rather than being a de novo primary malignant tumor was suggested in our patient because microscopic histology showed atypical or benign findings according to the above six histologic criteria but other clinical findings showed relatively rapid tumor growth and lymphatic and vascular invasion at 1 year follow-up.
In the treatment of gastrointestinal GCTs, benign lesions <2 cm in diameter and separated from the muscularis propria can be successfully removed by endoscopic resection; however, for large lesions (>2 cm) infiltrating the muscularis propria, surgery is the best therapeutic option for complete excision especially if the lesion was suspicious for malignancy.4,5,10 The prognosis of malignant GCT is considered poor; 30% to 40% of local recurrence and 50% to 60% of metastasis were reported in previous reviews; and the role of chemotherapy and radiation therapy is not yet verified.5,12
In summary, this case describes a submucosal tumor showing continuity to the third echo layer on EUS, and a rapid growth up to 3 cm was observed during the 1-year follow-up. The tumor showed histologically benign or atypical features but was diagnosed as a clinically malignant GCT with invasion of lymph nodes and vessels after segmental colon resection and lymph node dissection.
Notes
The authors have no financial conflicts of interest.