Endoscopic Management of Bile Leakage after Cholecystectomy: A Single-Center Experience for 12 Years
Article information
Abstract
Background/Aims
Bile leakage is an uncommon but serious complication of cholecystectomy. The aim of this study is to evaluate the efficacy of the endoscopic management of bile leakage after cholecystectomy.
Methods
A total of 32 patients who underwent endoscopic retrograde cholangiopancreatography (ERCP), because of bile leakage after cholecystectomy, from January 2000 to December 2012 were reviewed retrospectively. The clinical parameters, types of management, and procedure-related complications were documented.
Results
Most bile leakages presented as percutaneous bile drainage through a Hemovac (68.8%), followed by abdominal pain (18.8%). The sites of bile leaks were the cystic duct stump in 25 patients, intrahepatic ducts in four, liver beds in two, and the common bile duct in one. Biliary stenting with or without sphincterotomy was performed in 22 and eight patients, respectively. Of the four cases of bile leak combined with bile duct stricture, one patient had severe bile duct obstruction and the others had mild stricture. Concerning endoscopic modalities, endoscopic therapy for bile leak was successful in 30 patients (93.8%). Two patients developed transient post-ERCP pancreatitis, which was mild, and both recovered without clinical sequelae.
Conclusions
The endoscopic approach of ERCP should be considered a primary modality for the diagnosis and treatment of bile leakage after cholecystectomy.
INTRODUCTION
Postoperative bile leakage is a well-known, important complication after cholecystectomy, and when not detected may increase the morbidity and mortality rates.1,2 Currently, laparoscopic cholecystectomy (LC) is a well-established treatment for gallstone-associated diseases. However, the emergence of laparoscopic surgery has increased the incidence of bile leak, although it reduced the overall complication rate and length of hospital stays.3 Furthermore, the incidence of bile leak was reported to be higher after LC than after open cholecystectomy (OC), with an incidence of 1.1% to 4.0% after LC.4,5,6,7,8 The most common site of bile leak is the cystic duct stump, followed by the intrahepatic duct. The recent evolution of endoscopic therapy has played a major role in the diagnosis and treatment of bile leak after cholecystectomy. The aim of this study is to evaluate the efficacy of the endoscopic management of bile leakage after cholecystectomy.
MATERIALS AND METHODS
A total of 32 patients, including nine referrals from outside hospitals, with bile leakage after cholecystectomy from January 2000 to December 2012 were reviewed retrospectively. The exclusion criteria were bile leak after liver surgery, transarterial embolization, and traumatic injury. Bile leak was confirmed by means of endoscopic retrograde cholangiopancreatography (ERCP) in all patients. The medical records and endoscopic and radiologic findings were reviewed retrospectively. ERCP was performed, using side-viewing endoscopes (TJF-240; Olympus Optical Corp., Tokyo, Japan), by experienced endoscopists at a single center. Endoscopic retrograde biliary drainage (ERBD, Cotton-Leung stents; Wilson-Cook Medical, Winston-Salem, NC, USA) with or without endoscopic sphincterotomy (ES) or endoscopic nasobiliary drainage (ENBD, Wilson-Cook) was performed when the optimum cholangiogram showed findings of a definite bile leakage. The severity of the bile leak was categorized into low grade and high grade on the basis of fluoroscopic findings. A low-grade bile leak was defined as a leak identified simultaneously or right after the full opacification of the intrahepatic biliary trees fluoroscopically, and a high-grade leak was defined as the visualization of contrast extravasation before opacification of intrahepatic ducts. Patients were discharged in the absence of clinical evidence of a leak, and a second-look ERCP was usually performed after 4 to 6 weeks to confirm complete healing of the leak and to remove the biliary stent. Clinical parameters, including sites of bile leak, types of procedures, radiologic and endoscopic management, and ERCP-related complications, were documented. The study was approved by the institutional review board of our hospital.
RESULTS
Baseline characteristics
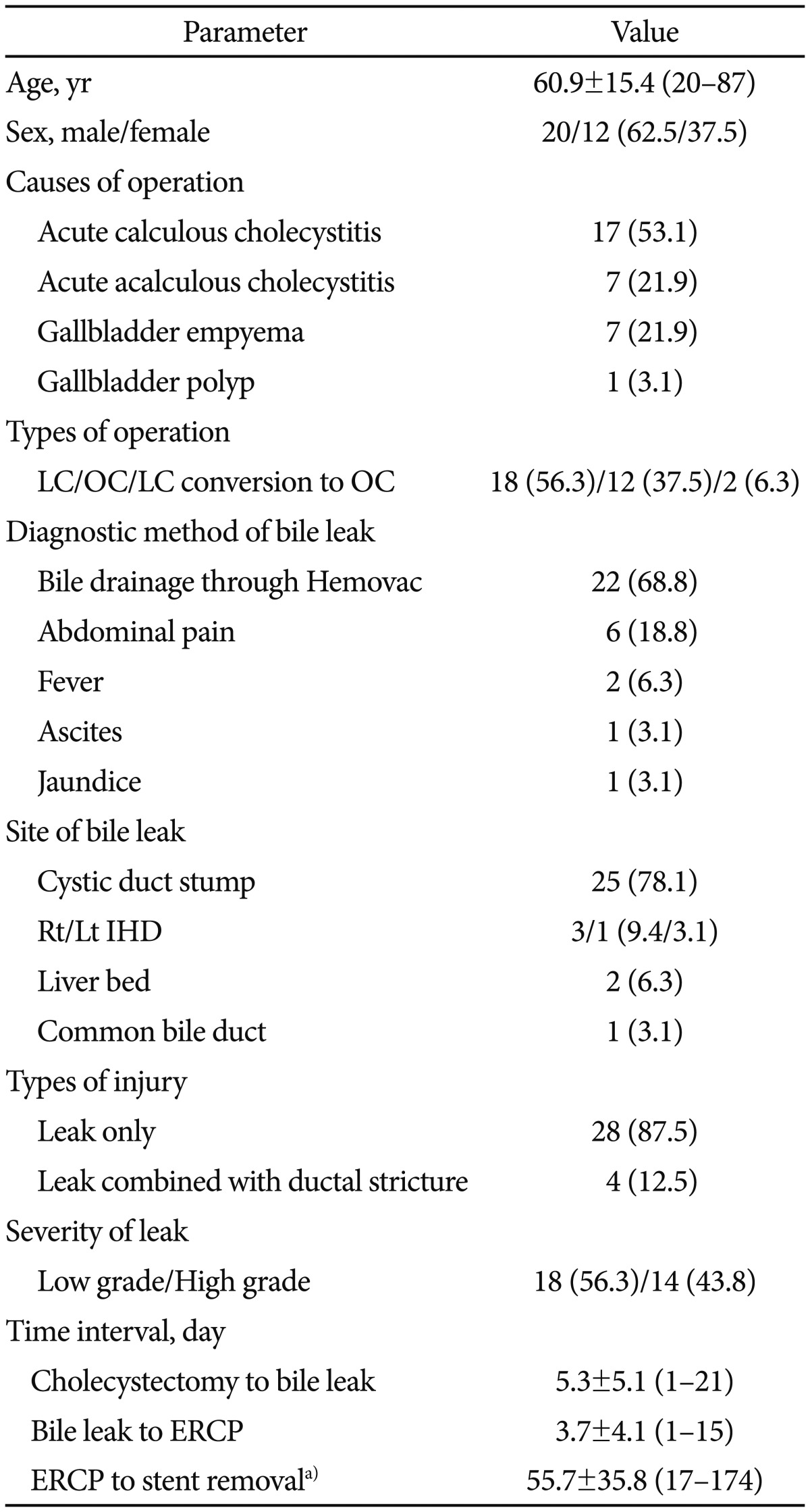
During the 2000 to 2012 period, >5,000 cholecystectomies were performed in our hospital. Twenty-three patients developed bile leaks after cholecystectomy at our hospital and nine patients with bile leak were referred from outside hospitals. Consequently, 32 patients (20 men, 12 women; mean age 60.9±15.4 years; range, 20 to 87 years) were included in the present study (Table 1). LC was carried out in 18 patients (56.3%), OC in 12 patients (37.5%), and two patients (6.3%) were converted to open laparotomy because of the technical difficulties associated with the laparoscopic approach. The causes of cholecystectomy were acute calculous cholecystitis (53.1%), acute acalculous cholecystitis (21.9%), gallbladder empyema (21.9%), and gallbladder polyps (3.1%). Of the 32 patients, 22 (68.8%) presented with persistent bile drainage through Hemovacs. The presenting symptoms included abdominal pain (18.8%), fever (6.3%), ascites (3.1%), and jaundice (3.1%). The most common site of bile leakage was the cystic duct stump (25 patients, 78.1%), followed by right hepatic duct (three patients, 9.4%), liver beds (two patients, 6.3%), left hepatic duct (one patient, 3.1%), and common bile duct (CBD; one patient, 3.1%). Of 32 patients with bile leak, four patients had bile duct stricture combined with bile leak, one of whom had a severe CBD stricture. On the basis of the severity of the bile leakage, 14 patients (43.8%) were classified as having a high-grade bile leak. The mean interval from cholecystectomy to the diagnosis of bile leak and from the diagnosis of bile leak to the referral for ERCP was 5.3±5.1 days (range, 1 to 21) and 3.7±4.1 days (range, 1 to 15), respectively. The mean interval from ERCP to stent removal was 55.7±35.8 days (range, 17 to 174).
Management of bile leakage and outcomes
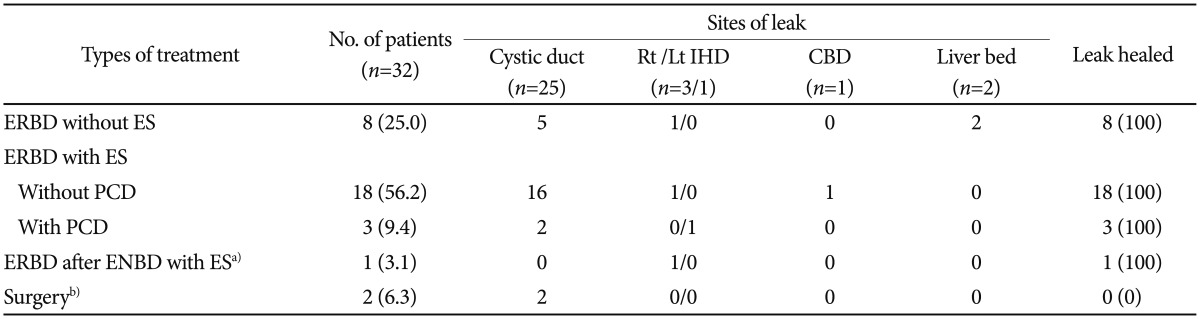
The types of procedures and endoscopic managements used for biliary leak are listed in Table 2. Various treatment modalities were performed. Twenty-seven patients (84.4%) were managed with ERCP alone and five (15.6%) were treated with a percutaneous intervention followed by ERCP. Of the 32 patients, endobiliary stent placement was performed after ES in 22 patients (68.8%) and without ES in eight patients (25.0%). One patient (3.1%) with gallbladder empyema underwent ENBD after ES, and follow-up cholangiography by means of ENBD demonstrated incessant extravasation of contrast at the right intrahepatic duct. The ENBD was replaced with a 10-Fr ERBD stent, which resulted in complete cessation of the bile leak.
In one patient, percutaneous catheter insertion was effectively attempted to drain the biloma at the gallbladder fossa; nevertheless, the patient experienced an acute respiratory distress syndrome attack and intracerebral hemorrhage associated with a septic condition, and finally complete healing of the bile leak was achieved with ERBD placement (Fig. 1). One male patient treated with percutaneous catheter drainage (PCD) insertion had an additional 10-Fr stent placed to attenuate the bile leak at the stump of the cystic duct. This patient required open laparotomy because of the persistent bile leak into the abdomen, although follow-up ERCP demonstrated complete closure of the previous leaking point. In the operation field, an ongoing bile leak was identified from the right hepatic duct, and eventually the patient underwent hepaticojejunostomy and fully recovered. Of the four cases of bile leak combined with bile duct stricture, one patient underwent hepaticojejunostomy after ERCP because of the massive bile leak combined with severe obstruction of the mid-CBD, and the remaining three patients were treated by means of ERBD with ES without further intervention because they had mild strictures.
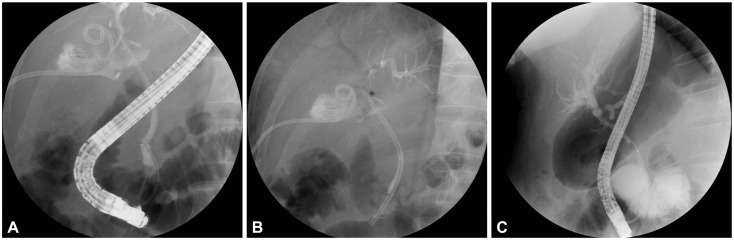
Endoscopic retrograde cholangiopancreatogram and follow-up cholangiogram of a cystic stump bile leak. (A) Extravasation of contrast was observed in the region of the cystic duct stump by cholangiography. Percutaneous catheter for the drainage of the biloma is also noted. (B) A plastic stent (10 Fr, 7 cm) was placed through the ampullary orifice. (C) No bile leak was evident after stent removal 6 weeks later.
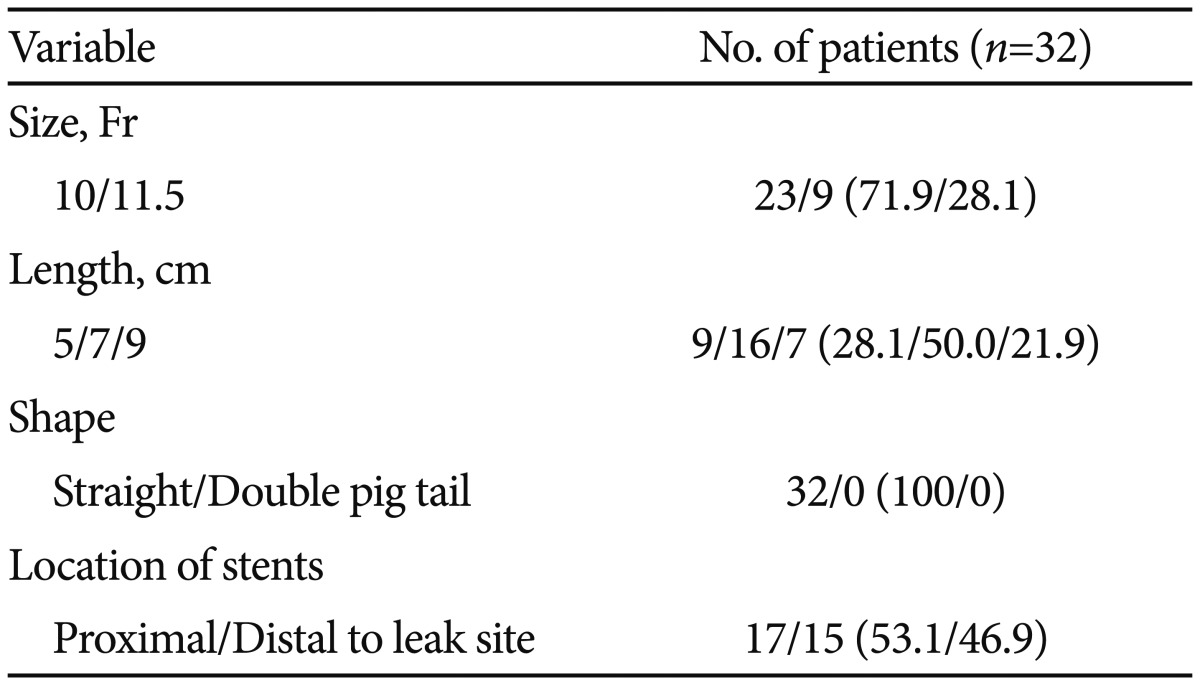
During follow-up, the ERBD stent spontaneously disappeared in four patients (12.5%). Endoscopic therapy with or without percutaneous intervention proved to be successful in 31 of 32 patients, with the exception of one patient who finally underwent surgery because of bile leak exacerbation. Table 3 outlines the sizes and lengths of the plastic stents used for the bile leaks. The diameters of the stents used for stopping the bile leak were 10 Fr in 23 patients (71.9%) and 11.5 Fr in nine patients (28.1%), and the lengths of stents were 7 cm in 16 patients (50.0%), 5 cm in nine patients (28.1%), and 9 cm in seven patients (21.9%). All ERBD stents used in this study were straight polyethylene stents.
Complications of endoscopic management
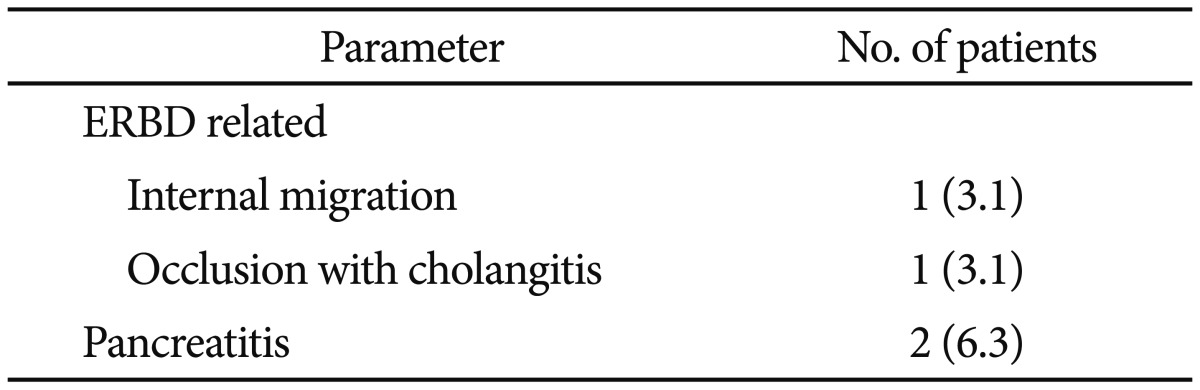
The procedure-related complications of the 32 patients who underwent ERCP for bile leak are listed in Table 4. Internal migration of the ERBD stent into the bile duct was encountered in one patient (3.1%). Stent occlusion complicated with acute cholangitis occurred in one patient (3.1%) with a 10-Fr stent. In this case, the ERBD stent was replaced with an 11.5-Fr stent, which subsequently resulted in the closure of the leak site. Two patients (6.3%) developed post-ERCP pancreatitis. Both these patients had ERBD without antecedent ES, one with a 10-Fr stent and the other with an 11.5-Fr stent. These patients had mild leaks and recovered without clinical sequelae. No complication associated with sphincterotomy was encountered.
DISCUSSION
Bile leakage is a well-known but uncommon complication after cholecystectomy. Since the introduction of LC for the treatment of gallstone disease, bile leakage has more frequently been encountered owing to unexpected injuries to the biliary tree. The incidence of bile leak seems to be higher for less experienced surgeons or at institutes with a low volume of laparoscopic surgery.1 Furthermore, it has been reported that bile leak is more frequent after LC than after OC, and the cystic duct stump is the most common site of bile leak, as was found in the present study.1,3 Other sites include the aberrant duct of Luschka, common hepatic duct, and intrahepatic ducts.1 In particular, variant bile ducts or accessory ducts of Luschka are especially vulnerable to accidental injury during cystic duct dissection. During cholecystectomy, iatrogenic dissection of an aberrant duct, erroneous clipping, dislodgement of clips, electrosurgical damage, ischemic change, and subsequent necrosis of a cystic duct stump can cause bile leakage.7,9,10 In this era of LC, surgeons should be aware of accessory ducts and of anatomical variations in the biliary tree in order to avoid iatrogenic injuries that could cause bile leakage.
The clinical symptoms of bile leak are diverse. In the present study, bile leak was diagnosed in most patients from percutaneous bile drainage (68.8%). Overall, abdominal pain (18.8%) was the most common symptom, followed by fever, ascites, and jaundice, which concurs with previous reports.2,5,6,7 Infrequently, bile leak presents as biloma (an extrahepatic collection of bile). In these cases, bile leak presents some time after surgery because it is not immediately noticed. Although many patients have been reported to develop symptoms 3 to 5 days after cholecystectomy, anecdotal reports indicate that symptoms may occur as late as 60 to 90 days.5,11 In the present study, the mean time between cholecystectomy and the detection of bile leak was 5.3 days (range, 1 to 21).7,12
Of the available modalities, radionuclide scintigraphy, computed tomography (CT), and ultrasonography (US) are noninvasive diagnostic tools for detecting bile leakage. In contrast, ERCP, PCD, and open laparotomy are diagnostic and therapeutic approaches.6,12,13,14 In some cases, percutaneous intervention and endoscopic procedures have been used to reduce the incidence of unnecessary open laparotomy.11 In the present study, four patients were successfully treated with a combined percutaneous and endoscopic approach, and only one patient eventually underwent open laparotomy because of a deteriorating bile leak.
Biloma is a serious complication after biliary surgery. Biloma formation after cholecystectomy can cause high morbidity and mortality unless treated timely, and usually requires aggressive intervention.15 Percutaneous drainage is an alternative to surgery in terms of preventing progression into sepsis due to bacterial infection of the fluid collection in the abdomen. The gallbladder fossa is the most common location of biloma formation and has been reported in 10% to 30% of affected patients.2,4 Biloma can easily be detected by CT or US and can be effectively drained by percutaneous catheter insertion under US guidance. Biliary decompression by means of ERCP should be carried out as a supplementary measure to ensure rapid biloma shrinkage, and the drainage catheter can be left in place until minimal drainage is observed.7 In the present study, one case of a well-demarcated biloma was encountered at the gallbladder fossa, and the biloma was successfully evacuated by means of radiologic intervention and subsequent endoscopic treatment.
Currently, ERCP plays an important role in the diagnosis and stoppage of bile leakage after cholecystectomy, with technical evolution. The sites of bile leak and the amount of leakage can be determined by direct visualization of contrast extravasation. Optimal cholangiogram of the biliary tree can also reveal the presence of CBD stones, bile duct anomalies, and bile duct stricture. In addition, ERCP can facilitate the endoscopic treatment of the bile leak. Available endoscopic managements for bile leak include ES, ENBD, ERBD with or without ES, or a combination of these modalities. Biliary stenting showed a more rapid resolution of leak than ES alone in a randomized and controlled study with a canine model.16 However, no consensus has been reached about the optimal endoscopic intervention for the management of bile leaks.3,5,11,17,18
Lowering the transpapillary pressure gradient through ampullary orifice and leak site bridging provides the theoretical basis for the healing process of bile leaks. ES alone can lead to equalization of the pressure gradient between the bile duct and the duodenum, which decreases flow resistance and diverts bile flow into the duodenum, consequently facilitating leak sealing.3,6 On the basis of the therapeutic rationale of decreasing the pressure gradient, it would appear that placement of a plastic stent offers a more predictable pressure gradient-lowering effect than ES does. Insertion of a biliary endoprosthesis can prevent the mucosal damage caused by bile contents through a bridging effect, and can divert bile flow preferentially toward the ampulla. As expected, biliary stenting was preferred to ES alone in several previous studies.6,10,14,19 Interestingly, even positioning of stents below the leak site showed equally encouraging results.11 This observation implies that the abolition of a transpapillary pressure gradient is the most influential factor related to the healing mechanism of bile leaks.
Sandha et al.18 proposed the algorithm of endoscopic treatment for bile leakage based on the fluoroscopic criteria. From their flowchart, ES alone may be sufficient to close the bile leak for low grade leaks. Meanwhile, biliary stent placement with or without sphincterotomy was advocated as the initial therapy for high grade leaks because ES alone may be insufficient to block the defect. Although biliary stenting combined with ES is not mandatory according to the proposed classification, ERBD with ES was more frequently performed than ES alone regardless of the degree of the leak in the present study, to facilitate the closure of the leak by rapid pressure lowering and bypassing of the leakage site.
Various sizes and lengths of stents are being used according to the clinician's preference. In the present study, usually short-length plastic stents (5 cm in 28.1%, 7 cm in 50.0%, and 9 cm in 21.9%) were placed, regardless of the leak sites, and favorable outcomes were achieved. Nevertheless, the optimal diameter and length of a biliary stent and the time required for the complete resolution of bile leaks have not been determined.18 Larger-caliber stents might be more efficient because they reduce the risk of occlusion and maximize bile flow through the ampullary orifice. In the present study, a 10-Fr stent was more frequently used than an 11.5-Fr stent (71.9% vs. 29.1%, respectively), and no differences in the therapeutic outcomes were found between the two stents.
Leaks are likely to persist in the presence of factors contributing to increase the intraductal pressure. Unnoticed CBD stones or dysfunction of the sphincter of Oddi (SOD) can aggravate bile leak by increasing the bile duct luminal pressure.9,20 In particular, ES plays an important role in patients with missed CBD stones or SOD by removing CBD stones and equalizing the pressure gradient between the bile duct and the duodenum.
Biliary stenting with or without ES and nasobiliary tube placement result in equally excellent outcomes in stopping bile leaks.1,18,21 There are controversies about the necessity of ES before placing a biliary stent. ES may be helpful for inserting a large-caliber stent easily and reducing the risk of pancreatitis. In the present study, two cases of mild procedure-related pancreatitis developed. A 10-Fr stent was placed in one patient and an 11.5-Fr stent in the other patient without a prior ES. The rates of pancreatitis associated with biliary stent placement are variable, and while some studies have reported no pancreatitis, others have reported several cases.7,11,12,21,22,23 Larger stents might be more effective for lowering the risk of luminal occlusion. However, the placement of a large-diameter stent without ES might increase the risk of pancreatitis. On the other hand, the placement of a smaller caliber stents could reduce the risk of pancreatitis but increase the possibility of clogging of stents. Furthermore, the proper duration of stent placement has not been definitively determined. Several studies have shown that bile leaks are usually sealed off within 1 week of stent placement, and that 4 to 7 weeks are required for complete closure of the leakage. Therefore, it is generally accepted that it is advisable to remove a plastic endoprosthesis 4 to 6 weeks later.5,7,11,13,19 In the present study, most prostheses were left in place for 4 to 6 weeks (mean, 55.7±35.8 days), and the follow-up ERCP demonstrated successful termination of the bile leak.
Nasobiliary tube (ENBD) placement offers an alternative treatment of bile leak by diverting the bile flow into the tube. In particular, this procedure may be useful for patients with coagulopathy, such as liver cirrhosis or chronic renal failure, because it can be performed without ES. Furthermore, the status of the bile leak can be visualized by using a cholangiogram through a nasobiliary tube. Although ENBD does not require follow-up ERCP after the healing of bile leakage, it is not commonly recommended because of its inconvenience and risk of accidental tube removal.7,9,18,21 In the present study, a 7-Fr ENBD stent was placed in only one patient with gallbladder empyema. However, ERBD was later performed in this patient because of a persistent bile leak on follow-up cholangiogram through the ENBD.
Most cases of bile leak can be treated endoscopically. However, an additional surgery or radiologic intervention may be required if bile leak is accompanied by severe biliary stricture or occlusion. Among the nine patients with bile duct stricture, six patients needed surgery because of complete occlusion of the bile ducts.24 In the present study, of the four stricture cases, one patient underwent open laparotomy because of a massive bile leak in conjunction with severe upstream bile duct obstruction (by surgical clips).
In the present study, the overall success rate of endoscopic intervention with or without percutaneous drainage for the healing of bile leak was 93.8%. In addition, no important procedure-related complications were encountered. The results obtained in this study provide evidence supporting that endoscopic therapy is an effective and safe modality for bile leakage after cholecystectomy. Nevertheless, large prospective studies are needed to determine the proper size, shape, and length of stents, owing to the limitations of the retrospective, small-sample-size, and single-center results.
In conclusion, bile leakage remains a major concern after cholecystectomy. Clinicians should be alert in detecting bile leakage as early as possible. The endoscopic approach of ERCP should be considered a primary modality for the diagnosis and treatment of bile leakage after cholecystectomy.
Acknowledgments
This study was supported by a Yeungnam University grant in 2012.
Notes
The authors have no financial conflicts of interest.