INTRODUCTION
Colonoscopy remains the current standard method for investigation of the colon and terminal ileum. Certain prerequisites should be met before the procedure for a successful and safe colonoscopic examination.1,2 The reason for the colonoscopy should be checked, as well as the patient's concurrent medication and general health condition. Furthermore, proper sedative and bowel-cleansing agents should be carefully selected for each individual patient (Table 1).
As proper bowel preparation is essential for a successful colonoscopic examination, the appropriate prescription and administration of the bowel-cleansing agent, as well as the patients' compliance, have already become important considerations. The choice of the bowel-cleansing agent needs to be individually tailored to the patient's condition. Moreover, there are many special conditions affecting bowel preparation that have to be also considered, such as advanced or pediatric age, pregnancy, lactation, renal or cardiac insufficiency, severe constipation, gastrointestinal (GI) bleeding, inflammatory bowel disease, and diabetes.3 In addition to proper mechanical cleansing methods, dietary modifications have proven to be effective when conducted concomitantly.3 The compliance of the patient to the preparation instructions also has been considered to be closely associated with the success of the procedure. Therefore, the importance of education regarding adequate bowel preparation has been emphasized.
As the use of anticoagulant and antiplatelet agents increases, their management has become more common and difficult to take into account during the periendoscopic period.
This review discusses the prerequisites and bowel preparation details that have to be considered before and during colonoscopy for an effective and safe examination.
MODULATION OF MEDICATION
As the elderly population grows, more patients receiving medications such as aspirin, anticoagulants, and nonsteroidal anti-inflammatory drugs (NSAIDs), are being referred to endoscopists for colonoscopy. For patient's convenience, polypectomy is often performed as soon as a polyp is detected to avoid another bowel preparation. Therefore, if the patient's concurrent medication increases the risk of bleeding after polypectomy, this should be considered before the colonoscopy. The patients in whom discontinuation of the antithrombotic agent poses only a low risk may stop their medication during the periendoscopic period.4,5,6 However, a careful evaluation is needed in cases when discontinuation of the antith-rombotic agent is associated with a high risk of adverse effects.4,5,6 A previous study showed that the use of aspirin or clopidogrel alone was not related to higher rates of postpolypectomy bleeding.7
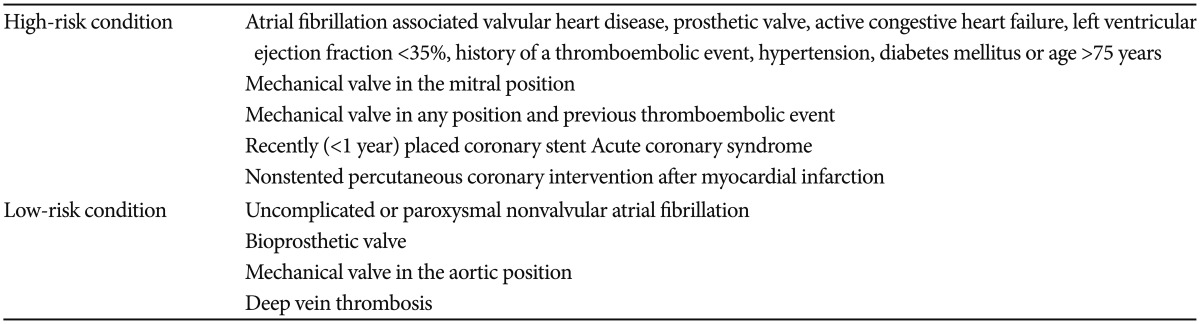
The management of the medications needs to be considered during the periendoscopic period in patients receiving anticoagulant agents such as warfarin, unfractionated heparin (UFH), and low molecular weight heparin (LMWH), and antiplatelet agents such as aspirin, NSAIDs, dipyridamole, thienopyridines (clopidogrel and ticlopidine), and glycoprotein II/IIIa (GP II/IIIa) inhibitors (tirofiban, abciximab, and eptifibatide).4,6 The management is based on the assessment of the procedure-related bleeding risk and potential thromboembolic risks related to the discontinuation of the medication.4,5,6
Aspirin and/or NSAIDs are recommended to be continued during all endoscopic procedures, and clinicians may discontinue aspirin and/or NSAIDs for 5 to 7 days before the high-risk procedures such as polypectomy and endoscopic submucosal dissection.4,6 In patients with a vascular stent or acute coronary syndrome, clopidogrel or ticlopidine may be withheld for 7 to 10 days before the endoscopy, provided that a minimum recommended period after the corresponding treatment has passed, and aspirin could be continued.4,6 If clopidogrel or ticlopidine is used for other indications, these medications could be continued for low-risk procedures such as diagnostic colonoscopy including biopsy.4,6 However, they need to be discontinued for 7 to 10 days before high-risk procedures.4,6 Anticoagulant (warfarin) discontinuation is recommended in patients with a low risk of thromboembolic events (Table 2).4,6
Continuation of anticoagulation by switching to LM-WH or UFH is recommended in the periendoscopic period in patients with higher risks of thromboembolic complications (Table 2).4,6 In patients with a high risk of thromboembolic events, UFH or LMWH needs to be restarted as soon as possible, and warfarin can be restarted on the day of the procedure without a significant danger of bleeding.4,6 In patients with a low risk of thromboembolic events, warfarin may be restarted on the evening after the endoscopy without a high risk of postprocedural bleeding.6
In patients with acute GI bleeding receiving an anticoagulant or antiplatelet agent, this medication is recommended to be withheld until hemostasis is achieved.6
ENDOSCOPIC SEDATION
The purposes of procedure-related sedation include safe and effective management of pain and anxiety in addition to acquirement of a proper degree of memory loss and decreased awareness. Currently, there is no standard regimen regarding sedation in GI endoscopy.8 The choice of sedation may differ depending on the endoscopist's preferences and the type of planned procedure. In special conditions such as obesity, pregnancy, advanced age, and chronic lung, liver or renal disease, special considerations and precautions are required regarding the dose adjustment and choice of sedative drugs.8,9
Midazolam is considered the benzodiazepine of choice as it provides a shorter duration of action with a better pharmacokinetic profile than diazepam.8,9 Pethidine and fentanyl are the most popular opioids.8,9 Reversal drugs for endoscopic sedative drugs consist of flumazenil and naloxone.9 Flumazenil, a benzodiazepine antagonist, reverses the respiratory and sedative effects of benzodiazepine.9 Naloxone, a pure mu-opioid antagonist, reverses both the respiratory and analgesic effects of opioids.8,9
Unsedated endoscopic procedures are recommended for elderly patients or patients with the risk of cardiopulmonary dysfunction.
The use of propofol for sedation during diagnostic and therapeutic procedures is increasing as it enhances the quality of upper GI endoscopy by increasing the patient's acceptance of the procedure and the diagnostic accuracy.10 In addition, it has satisfactory sedative, hypnotic, antiemetic, and amnesic properties, as well as a rapid onset of action and a short recovery profile.8,9 Its use is preferred in patients with advanced liver disease because of its short biologic half-life resulting in a low risk of hepatic encephalopathy.9 With regard to side effects, propofol may induce cardiopulmonary events.8,9 It can cause a dose-dependent decrease in cardiac contractility leading to a decrease in cardiac output, systemic vascular resistance, and arterial pressure.8,9 In addition, it may be associated with serious adverse events such as respiratory depression, airway obstruction, and death.8,9 Unfortunately, there is no pharmacological antagonist for this compound.8,9 In a prolonged and potentially uncomfortable endoscopic procedure, intravenous midazolam along with propofol for sedation has been reported to be more effective than intravenous midazolam alone, without differences in the safety.11
Meperidine (category B) followed by small doses of midazolam (category D) as needed is recommended for moderate sedation in endoscopic procedures during pregnancy.12 Breast-feeding may be continued after fentanyl (category C) or propofol (category B) administration in lactating patients after sufficient recovery from general anesthesia.12 Infants should not be breastfed for at least 4 hours after midazolam is administered to the mother.12
Patient's age, inpatient status, higher American Society of Anesthesia grade, routine use of oxygen, and trainee participation were associated with a higher incidence of unplanned cardiopulmonary events during GI endoscopy under conscious sedation.13
DIET
Although dietary modifications alone are not sufficient for preparation for colonoscopy, they have proven to be effective when conducted together with mechanical cleansing.3 For dietary regimens, clear liquids and low-residue diets are recommended for 1 to 4 days before colonoscopy.3,14 Patients are allowed to have water, clear soup, clear fruit juice without pulp, coffee or tea without milk, and sport drinks on the clear liquid diet.14 In addition, patients may have white rice, white rice cakes, refined noodles or pasta, vegetable juices, grapes without skin and seeds, peaches without skins and seeds, watermelon without seeds, well-cooked potatoes without skin, tender meat, fish, chicken, and eggs on the low-residue diet.14 Patients are forbidden to have high-fiber foods such as brown rice, whole grains, raw and dried fruits, seeds, nuts, and multigrain bread.14 Prolonged dietary restrictions may also be an important factor for better colon preparation, but they could lead to lower compliance.3 Nevertheless, prolonged fiber restriction with liquid diet needs to be suggested in cases of severe constipation.15 Furthermore, a study suggested that the fiber-free diet is more effective than the clear liquid diet if it is combined with the use of polyethylene glycol (PEG) electrolyte solution on the day before colonoscopy.16
BOWEL PREPARATION
The ideal preparation for colonoscopy needs to satisfy the requirement of emptying the colon of all solid or liquid materials in a rapid fashion with no gross or histological changes in the colonic mucosa. Additionally, shifts in fluids or electrolytes, patient discomfort, and cost should be kept to the minimum.17
PEG-electrolyte lavage solution is the most frequently prescribed bowel-cleansing agent. As it is a nonabsorbable solution, it passes through the bowel without net absorption or secretion, and significant fluid or electrolyte shifts do not occur.3 Therefore, PEG is considered safer than stimulant laxative/sodium phosphate (NaP) in patients with fluid or electrolyte imbalance.3 It is preferred in patients with renal insufficiency, congestive heart failure, or liver failure.3 The drawbacks of this agent are that it should be diluted in a large volume of water (up to 4 L) to reach the desired cathartic effect and it's unpalatable taste despite flavoring, which leads to poor compliance.3 Sulfate-free PEG (SF-PEG) was developed to improve the taste and smell of the PEG solution by decreasing the potassium concentration, increasing the chloride concentration, and eliminating sodium sulfate.3 SF-PEG is considered to be comparable to PEG in safety, effectiveness, and tolerance, but it still requires consumption of 4 L of the diluted agent.3 Aqueous NaP is no longer prescribed, as it may cause significant fluid and electrolyte shifts resulting in renal failure; however, NaP tablets are still available.
Adjunctive agents are used to enhance the cleansing efficacy of bowel preparation conducted by the main purgative regimens such as PEG, as well as to reduce the volume of fluid that needs to be taken to achieve a cathartic effect.18 Ascorbic acid, which is not completely absorbed and remains in the colonic lumen, exerts an osmotic effect and is used with a smaller quantity of PEG.19,20 Low-volume PEG solutions with ascorbic acid have been reported to be comparable to high-volume PEG solutions in efficacy and tolerability by the patients.19,20 Magnesium salts that show a synergic effect through their osmogenic properties are often used with picosulfate, a prodrug that is metabolized to a peristalsis-enhancing stimulant within the bowel lumen.21 The regimen with sodium picosulfate and magnesium citrate is gradually accepted as a major bowel-cleansing regimen based on its efficacy and safety profiles.22 Other adjuncts such as bisacodyl, senna, and metoclopramide have been reported to have the advantage of reducing the volume of the solution required for bowel cleansing; however, their exact efficacies and safety profiles remain to be established.3,18,23
A meta-analysis found that a divided-dose PEG solution regimen (initial 2 to 3 L is given the night prior to the colonoscopy and the remaining 1 to 2 L on the morning of the procedure) improves the quality of bowel preparation, increases patient compliance, and reduces the incidence of nausea that leads patients to discontinue bowel preparation when compared with full-dose PEG.3,24,25
The quality of bowel preparation may be influenced by the interval between the end of the preparation procedure and the start of colonoscopy.26 It is suggested that colonoscopy needs to be performed within 7 hours from the start of PEG intake and 4 hours from the end of PEG intake to improve the quality of bowel preparation.26 If colonoscopy is scheduled in the afternoon, bowel preparation may be carried out on the same day, resulting in better feasibility, safety, and effectiveness, as well as fewer adverse events, and leading to patients' preference.26,27
Elderly patients tend to show higher rates of inadequate colon cleansing for colonoscopy.28 A dietary restriction is helpful, with clear liquids and low-residue diets for 1 to 4 days prior to the colonoscopy.15 Moreover, cleansing by PEG consumption <5 hours prior to colonoscopy is efficient.15
In patients with severe constipation, a longer period of staying on a liquid diet, application of alternating bowel-cleansing agents, use of an adjunctive laxative, and use of a double dose of the PEG solution are recommended for successful bowel preparation, as they have increased colon transit time and may be resistant to laxatives.15
In diabetic patients, inadequate bowel cleansing has been reported, as they have increased colon transit time and constipation.15 NaP should be avoided in diabetic patients because of the potential risk of hyperphosphatemia and metabolic acidosis with impaired renal function.15 In addition, NaP should be avoided in patients with congestive heart failure and renal dysfunction, as well as in patients with medications affecting renal function such as diuretics, angiotensin receptor blockers, and angiotensin-converting enzyme inhibitors.15,29 In addition, sodium picosulfate must be avoided in individuals with a risk of hypovolemia, such as patients with congestive heart failure, advanced liver cirrhosis, and chronic kidney disease, as well as patients taking high doses of diuretics.30
Patients with stroke may have difficulties swallowing, and patients with dementia may have difficulties taking large amounts of fluid.15 The bowel preparation solution may be administered directly into the stomach or duodenum through an esophagogastroduodenoscope using a water irrigation pump or nasogastric tube.3,15,29
NaP or sodium picosulfate/magnesium sulfate regimen needs to be avoided in patients with suspected inflammatory bowel disease, as such drugs may cause mucosal abnormalities that present symptoms mimicking colitis.15,31
In patients with lower GI bleeding, adequate bowel preparation may be beneficial for the identification of the bleeding source.15 If the amount of bleeding is suspected to be small, bowel preparation using PEG solution may be helpful. However, enema is preferred if the bleeding source is presumed to be within the rectal area, or the amount of bleeding is suspected to be severe.15,32
In pediatric patients, the most widely accepted regimen for bowel cleansing is 1.25 mg/kg PEG administration over a 4-day period combined with a liquid diet given on the fourth day.21 PEG solution may be delivered through a nasogastric tube if the pediatric patient cannot tolerate oral ingestion.15
In pregnant patients, endoscopy should be performed only when strongly indicated and must be postponed until the second trimester.12 Although both PEG and NaP solutions are pregnancy category C drugs, PEG is preferred over NaP as a small amount of PEG may safely control constipation in pregnancy.3 When cathartic agent administration or tap water enema is performed, breastfeeding may be interrupted as a precaution.29
EDUCATION
Inadequate bowel preparation is associated with a decrease in the cecal intubation rate, increased risk of missing important lesions, increase in the number of cancelled procedures, prolonged procedure time, and higher risk of complications.1,2,3 Appropriate bowel preparation is closely related to the compliance of the patient to the preparation instructions. Therefore, patients' understanding of colonoscopy and bowel preparation may influence the outcome of the procedure. One study suggested that non-compliance with bowel preparation instructions and lower education level were independent risk factors for poor bowel preparation.33 Education of patients is considered a very important factor to ensure compliance before colonoscopy, and many studies have suggested diverse education programs that have resulted in apparent increases in patient compliance.34,35,36,37,38 Nurse-delivered education with brochures, an educational pamphlet, a novel patient educational booklet, and cartoon visual aids were suggested to be effective in increasing the quality of bowel preparation.34,35,36,37 There has also been a study showing that telephone-based re-education regarding the details of bowel preparation on the day before colonoscopy enhanced the quality of bowel preparation as well as the polyp detection rate.38
CONCLUSIONS
For effective and safe colonoscopy, proper prerequisites and appropriate bowel preparation should be achieved and verified before and during the procedure. Modulation of antithrombotic agents should be considered based on the procedure-related bleeding risk and potential thromboembolic risks related to the discontinuation of the medication. The proper choice of sedation and bowel-cleansing regimen, together with diet modifications, should be based on the patient's underlying disease, age, and medication intake. Finally, it is important to develop effective methods of patient education regarding bowel preparation.