INTRODUCTION
When endoscopic therapy for dysplastic Barrett esophagus first became a possibility, the management strategy seemed easy. Barrett esophagus predisposed to esophageal adenocarcinoma (EAC), and cure without esophagectomy was finally possible when this cancer was detected before the development of symptoms such as dysphagia, which heralded a poor prognosis. Because of the long latency period before Barrett esophagus becomes neoplastic, a gastroscopy was recommended for anyone suspected of having a Barrett esophagus. However, more recent studies have suggested that while the relative risk of developing EAC from Barrett esophagus may appear an impressive statistic, the absolute risk may be smaller than previously suspected. The benefits of screening, the cost and burden of gastroscopy, and the subsequent management remain to be established by a prospective randomized trial. The literature is increasingly recognizing that the value of traditional endoscopy for screening and surveillance of Barrett esophagus may be more limited than initially believed. For example, in a retrospective case-control study of patients with EAC on a background of Barrett esophagus, endoscopic surveillance did not change mortality from EAC.1 Furthermore, only a few cases of EAC develop in previously detected Barrett esophagus, and in a population of patients with Barrett esophagus, death from EAC was dwarfed by mortality from ischemic heart disease.2,3 With this in mind, we also know that the population at risk of Barrett esophagus is large, and the rates of EAC are rising worldwide. No consensus has emerged on when to perform upper endoscopy for detection and surveillance of Barrett esophagus. The future of deciding when to screen a patient for Barrett esophagus may lie in the more precise identification of high-risk individuals and the utilization of more cost-effective screening methods.
The management of dysplasia in Barrett esophagus and the indications for endoscopic surveillance in this condition are not discussed in this article.
ESOPHAGEAL CANCER AND BARRETT ESOPHAGUS
Esophageal cancer is the eighth most common cancer and the sixth most common cause of cancer death worldwide. It was responsible for an estimated 400,000 cancer deaths in 2008, and its incidence continues to rise.4,5 Significant geographical differences exist in the rates of the two types of esophageal cancer: esophageal squamous cell carcinoma (ESCC) and EAC. Prognosis in both ESCC and EAC remains poor with 5-year survival rates between 13% and 23%, although this appears to be improving. Poor outcomes primarily relate to the frequent occurrence of advanced metastatic stage at diagnosis. Prognosis is better in earlier stage disease.5-11 In the East, the predominant type of esophageal cancer is ESCC, which has been associated with alcohol consumption, tobacco use including active and passive smoking, high intake of pickled foods, low intake of fresh fruit and vegetables, low socioeconomic status, poor oral hygiene, frequent consumption of extremely hot drinks, caustic injury, radiation, and achalasia.7,12-15 As opposed to the increasing rates of ESCC in the East, the rates in the West are decreasing, following a reduction in smoking and alcohol consumption.
In much of the West, EAC is becoming the predominant type of esophageal cancer. The rate of EAC has been rising rapidly in Australia, North America, the United Kingdom, and Western Europe, with increases in both sexes. EAC is a male-predominant cancer, with a male to female ratio of 3 to 9:1 in Western countries.16-22 EAC is now responsible for more than half of the esophageal cancer cases in the United Kingdom and the United States.
The precursor to EAC is postulated to be Barrett esophagus with a progressive sequence through low-grade to high-grade dysplasia (HGD), intramucosal carcinoma, and invasive EAC,23,24 although this progression is by no means inevitable. Barrett esophagus is the metaplasia of squamous-lined esophagus to a columnar lined esophagus. This change begins at the gastroesophageal junction (GEJ), and as such, the identification of the GEJ is crucial to the diagnosis of Barrett esophagus. In much of the West, the GEJ is defined as the proximal ends of the gastric rugae. In the East, the definition of the GEJ is often taken as the distal end of the longitudinal esophageal mucosal vessels. Metaplasia of the esophagus with a gastric type lining is also variably classified as Barrett esophagus.
A main risk factor for the development of Barrett esophagus is gastroesophageal reflux disease (GERD), often associated with a hiatal hernia. The risk is likely related to the severity of the reflux and its duration.25 Barrett esophagus is also associated with smoking, increased body mass index, abdominal obesity, and possibly a reduction in Helicobacter pylori infection.26-30
Advances in endoscopic treatments for dysplastic Barrett esophagus have opened the possibility of EAC screening, but numerous factors must be taken into account in the assessment of whether screening for EAC is justified or to whom it should be offered. For cancer screening to be possible, general principles require that a precancerous stage is detectable with a clinically significant risk of cancer.31 Treatment at this stage should then be able to significantly alter the natural history of the disease and should be cost-effective to the community. A comprehensive understanding of the epidemiology and natural progression of Barrett esophagus and EAC, as well as the benefits, costs, and other burdens of intervention is now needed.
Incidence and prevalence of Barrett esophagus
The prevalence of Barrett esophagus differs with geographical location and is higher with increasing age, male sex, and the presence of risk factors. In a well-conducted population prevalence study from Sweden, the risk of Barrett esophagus was approximately 1.2% in asymptomatic individuals, increasing to 2.3% in those with reflux symptoms.32 In a general practice setting in the United Kingdom, patients aged 50 to 70 years with a history of prescription for acid suppressing medication had a 3% prevalence of Barrett esophagus.33
In populations undergoing upper endoscopy for various reasons, the prevalence may be higher. An analysis of a North American multicenter endoscopy database found that the risk of Barrett esophagus in white men with reflux symptoms rose from 3.3% at age 30 to 40 years to 6.3% at age 40 to 50 years and 9.3% at age 50 to 60 years before plateauing. For white men without reflux symptoms, 2.4% aged 40 to 79 years had Barrett esophagus. This risk was similar to white women with reflux symptoms. Less than 1% of white women without GERD were found to have Barrett esophagus regardless of age.34
The prevalence of Barrett esophagus seems to be increasing in Asian countries such as Japan, Korea, Malaysia, and Singapore based on hospital studies.35-37 In Korea, one retrospective review of 6,683 patients undergoing screening upper endoscopy as part of the National Cancer Screening Program (NCSP) revealed that the rate of erosive reflux esophagitis was 8.45% and the rate of Barrett esophagus was 1.1%.38 In another Korean hospital-based retrospective review of 4,002 patients presenting for screening upper endoscopy, the prevalence of Barrett esophagus was 1%.39 In a large prospective study of 25,536 subjects undergoing upper endoscopy screening at 40 Korean hospitals during a 7-month period, the prevalence of Barrett esophagus confirmed through histology was 0.84%.40
PROGRESSION OF BARRETT ESOPHAGUS
Progression from nondysplastic Barrett esophagus to esophageal adenocarcinoma
Current endoscopic surveillance guidelines are predominantly based on the assumed risk of EAC developing in nondysplastic Barrett esophagus at approximately 0.5% per year. This figure was defined in a meta-analysis more than a decade ago.41,42 A more recent meta-analysis of studies with stricter inclusion criteria reported incidence rates for the development of EAC in nondysplastic Barrett esophagus of 0.33% per year and 0.19% for short segment Barrett esophagus.43
Recent large population-based studies have reported even lower rates of progression. A study using a database of histology results from all of Northern Ireland (1.7 million) reported a risk of progression from nondysplastic Barrett esophagus to EAC of 0.13% per year, or 0.18% per year for well defined Barrett esophagus (specialized intestinal metaplasia and visible segment). In the same study, the combined annual incidence of HGD, EAC, and gastric cardia cancer was, however, still 0.33%.44 A retrospective study based on prospectively collected pathological data from the whole population of Denmark (5.4 million) found the risk of progression from nondysplastic Barrett esophagus to EAC to be 0.12% per year (95% confidence interval [CI], 0.09 to 0.15). In this study, a prior diagnosis of nondysplastic Barrett esophagus gave a relative risk of developing EAC that was 11.3 times that of the general population.45
Other well-conducted studies show higher rates; however, and it is unlikely that the Danish registry data can be generalized worldwide. For example, a large prospective Dutch cohort study of more than 16,000 participants showed an annual incidence rate of 0.4% for developing EAC in nondysplastic Barrett esophagus and 0.58% for progressing to a combination of HGD or EAC even excluding cases if progression occurred within 12 months of diagnosis.46
A progression rate of 0.12% is considerably lower than the previously assumed 0.5%, which has important follow-on implications for assessing the cost effectiveness of Barrett esophagus surveillance. Many factors seem to influence the rates of progression found in different studies. These factors include the criteria used for identifying Barrett esophagus, in particular histological alone versus histological plus endoscopic findings, the accuracy of pathological assessment, and the underlying risk factors for progression inherent to each population group. In summary, while different studies do give varying results, the progression of nondysplastic Barrett esophagus to EAC seems to be between 0.12% and 0.5% per year.
Progression from low-grade dysplasia to high-grade dysplasia and esophageal adenocarcinoma
The risk of progression from low-grade dysplasia (LGD) to more advanced dysplasia or EAC is unclear from the literature at present. This is probably in large part because of the imperfect nature of the histological method for assessing LGD, which suffers from low interobserver agreement. Studying the risk of progression from LGD is also heavily reliant on the exclusion of initially missed cases of HGD/EAC.
In the Danish cohort, 0.5% without LGD and 2.3% with LGD progressed to EAC after the first year of follow-up. The incidence of EAC in patients without LGD was 0.1% versus 0.51% for LGD (relative risk 4.8 for LGD).45 In the previously mentioned Dutch cohort study, of 16,333 patients with Barrett esophagus, there was a 6% progression to HGD or EAC without baseline dysplasia and 13% progression with baseline dysplasia at the 10-year follow-up (p<0.001).46 Independent risk factors for progression on multivariate analysis were male sex, older age, and the presence of LGD at initial diagnosis. Another Dutch trial identified LGD as a risk factor for progression along with a disease duration of more than 10 years, length of Barrett mucosa, and persistent esophagitis.47
High rates of progression from LGD to HGD or EAC have been reported, in particular when expert pathologists confirm the diagnosis of LGD. In a study of 147 patients with Barrett esophagus and LGD whose slides were reviewed by expert pathologists, 85% were down-staged to nondysplastic Barrett esophagus; however, in those where the diagnosis was confirmed, the risk of progression to HGD or EAC was 85% after a duration of 109 months.48 In a multicenter prospective cohort of 713 patients with Barrett esophagus, the absence of any risk factor in nondysplastic Barrett esophagus implied a risk of <1% for progression, whereas those with LGD and one other risk factor had a risk of progression of 18% to 40%.47
In a study challenging the data on the risk of progression, 210 patients with Barrett esophagus were followed for just over 6 years. After 12 months, there was no difference in the risk of progression to HGD or EAC in those with LGD as compared to those without LGD (12 patients progressed in each group, absolute risk 2.33% vs. 2.69%).49 In the same study, however, those with multifocal LGD as opposed to unifocal LGD had an increased incidence of EAC, 1.89% vs. 0.27%, which trended to significance (p=0.08). Also illustrated in this study was the difficulty in obtaining a consistent diagnosis of LGD by pathology. In those biopsies that were reviewed by two expert pathologists, the interobserver agreement was only 55.6% with a ╬║ value of 0.14.49
Importantly, smoking has been associated with an increased risk of progressing from Barrett esophagus to more advanced dysplasia and EAC. An analysis of smoking in the Northern Ireland registry showed a hazard ratio of 2 between current smokers and never smokers.50
Progression from high-grade dysplasia to esophageal adenocarcinoma
The rate of progression from Barrett esophagus with HGD to EAC is high. A meta-analysis of studies following patients with HGD revealed a high risk of progression to EAC. With a total of 236 participants, the risk of progression was 6.58% per year (95% CI, 4.97 to 8.9).51 In a randomized study of radiofrequency ablation in dysplastic Barrett esophagus, four of 21 patients (19%) with HGD in the sham therapy group progressed to EAC at 12 months.52
ENDOSCOPIC SCREENING FOR BARRETT ESOPHAGUS
In Korea, to reduce high gastric cancer mortality, as part of a comprehensive 10-year plan for cancer control, the NCSP was initiated in 1999. The NCSP recommends that men and women aged Ōēź40 years undergo upper endoscopy or upper gastrointestinal (UGI) series every 2 years.53 A recent publication has shown that with this program, the cost per 1 year of life saved from gastric cancer is approximately US $8,750 to US $9,680 for endoscopy and US $14,350 to US $14,900 for UGI series.54
In this program, assuming that endoscopy rather than UGI series was the method chosen for screening, screening for Barrett esophagus is offered to the entire Korean population aged Ōēź40 years.
One way that proposed screening could be analyzed is by assessing its cost effectiveness. One model demonstrated a cost of $10,440 per quality-adjusted year of life saved by screening of 50-year-old white men with reflux symptoms (assuming a Barrett esophagus prevalence of 10%). However, this analysis was based on assuming a progression rate of 0.5% per year from nondysplastic Barrett esophagus to EAC, which may be an overestimation.42 In fact, one recent study using a more conservative rate of progression from nondysplastic Barrett esophagus to EAC (0.15% per year) found that the cost of screening with endoscopy was $22,200 per year of life saved for a population of 50-year-old white men with a history of reflux (assumed prevalence of Barrett esophagus 8%).55 Thus, with endoscopy as the screening method for Barrett esophagus in a population with a moderately high prevalence of Barrett esophagus (8% to 10%), the costs of screening to save 1 year of life may be closer to $22,200 than $10,440.42,55
Identifying a population to screen
Efforts to identify a population to screen for Barrett esophagus have predominantly focused on patients with GERD and symptom duration and frequency as an indication of who to screen endoscopically.56 Although understanding these factors may be helpful in identifying patients with a significant chance of having Barrett esophagus, the majority of patients with EAC actually do not report previous symptoms of GERD.57 Recently, there has been an initiative to more accurately identify individuals at increased risk of having Barrett esophagus (bearing in mind that endoscopic screening itself has not necessarily been shown to be cost-effective or successful in reducing mortality).
Rubenstein et al.58 recently developed a risk calculation tool (Michigan Barrett Esophagus pREdiction Tool, M-BERET) based on a population of men aged 50 to 79 years presenting for colorectal cancer screening who were invited to undergo upper endoscopy at the same time. The population was predominantly white (89%) and overweight. A logistical model was created based on the four strongest independent predictors for Barrett in the studied group: age, waist hip ratio, GERD frequency, and cigarette use. The receiver operator characteristic (ROC) curve for the M-BERET tool as compared to a GERD model using GERD symptoms alone was a better predictor of the likelihood of Barrett esophagus (area under the ROC curve 0.72 vs. 0.6). At a sensitivity of 80%, the tool had a specificity of 56%. In comparison, in order for the GERD model to have a sensitivity >46%, all patients needed to be selected with a specificity of 0%. Of course, this tool has only been developed in a population of predominantly white overweight men with a significant history of cigarette smoking and is yet to be validated.
GERD is still considered a strong risk factor for developing Barrett esophagus, and an understanding of GERD-specific risk factors for Barrett esophagus is useful. A recent study limited to a veteran's affairs population undergoing elective endoscopy in the United States showed that the two factors with the highest odds ratio for predicting Barrett esophagus were a younger age of reflux onset (<30 years) and frequent GERD symptoms (Ōēźweekly).59
Ongoing research and the creation of good risk calculation tools will be essential in defining at risk populations, if screening for Barrett esophagus is to be entertained.
Novel screening methods for Barrett esophagus
Ideally, a screening test should have a high sensitivity for detecting Barrett esophagus and would be relatively inexpensive and easy to administer. Traditional endoscopy, although perhaps the gold standard for the diagnosis of Barrett esophagus, is somewhat limited by its cost effectiveness. Transnasal endoscopy has advantages over traditional endoscopy in that it can be performed without sedation, and thus, costs can be greatly reduced. A randomized trial comparing transnasal versus traditional endoscopy for the detection of Barrett esophagus showed essentially equivalent rates of detecting nondysplastic Barrett esophagus, although the biopsy specimens were smaller with transnasal endoscopy. Importantly, participants preferred transnasal endoscopy.60
A meta-analysis of capsule endoscopy compared with traditional endoscopy for the detection of Barrett esophagus showed a pooled sensitivity of 78% for the diagnosis of Barrett esophagus as compared to traditional endoscopy.61 The authors concluded that results were insufficient to recommend capsule endoscopy as a screening tool. Regardless, capsule endoscopy is unlikely to be less expensive or easier to perform than traditional endoscopy.
The Cytosponge (Cambridge University, Cambridge, UK) is a novel method for diagnosing Barrett esophagus and one that is both easy to perform and potentially economical. This nonendoscopic method involves swallowing an ingestible gelatine capsule attached to a string. The capsule dissolves after 5 minutes and releases a special sponge that is then pulled out from the gastric cardia, collecting cytological specimens on its passage through the GEJ and esophagus. The specimen is then analyzed for a cellular marker only present on intestinal cells (Trefoil factor 3) and is therefore in this setting a diagnostic biomarker for Barrett esophagus.62 In a study of 504 participants aged 50 to 70 years with a previous prescription of acid suppressing medication, 99% successfully swallowed the Cytosponge pill. Compared with traditional endoscopy, the Cytosponge had a sensitivity of 90% for diagnosing Barrett esophagus segments Ōēź2 cm in length and a sensitivity of 73.3% for a circumferential segment Ōēź1 cm in length.33 The cost of the Cytosponge compared to endoscopy has been assessed recently in a theoretical model, where a population with a Barrett esophagus prevalence of 8% was screened with the Cytosponge, followed by treatment of patients with dysplasia or intramucosal cancer at subsequent endoscopy. The costs of screening with the Cytosponge versus not screening was an additional $240 per screening participant and resulted in a cost of $15,700 per quality-adjusted year of life saved. The costs were compared to endoscopic screening, which had an additional cost of $299 per screening participant for $22,200 per quality-adjusted year of life saved.55 The costs of the Cytosponge could theoretically be reduced even further if another biomarker to screen for dysplasia was incorporated into the Cytosponge test, thus reducing the amount of endoscopy required for surveillance. Based on the authors' estimates, the uptake of the Cytosponge screening would reduce the number of cases of incident adenocarcinoma by 19% as compared with 17% for endoscopy.
WHEN TO PERFORM UPPER ENDOSCOPY
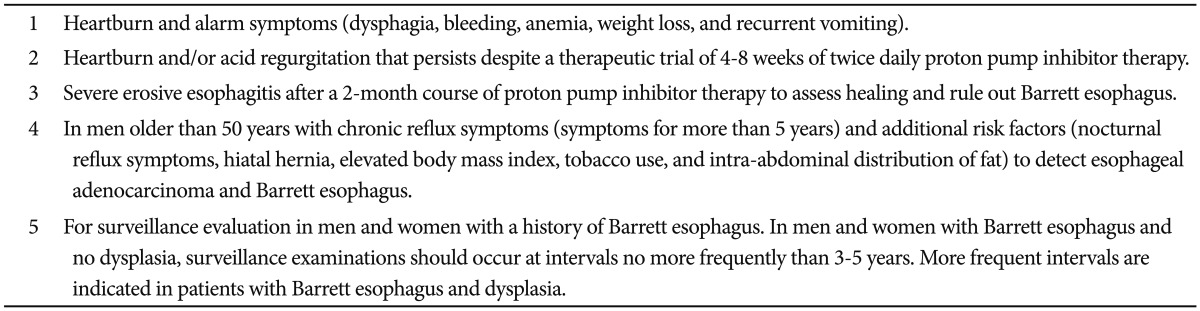
The benefits of performing an upper endoscopy for EAC prevention need to be considered from the perspective of the individual patient as well as the value and cost to the community. The question of who should undergo upper endoscopy also ties in with issues of diagnosing reflux esophagitis and determining its severity in many patients. Multiple expert physician groups have issued opinions on this matter. Although these guidelines do not fully answer the question of when to perform an upper endoscopy for Barrett esophagus, two are summarized in the tables below (Tables 1, 2).63,64 For asymptomatic patients, the decision of when to perform an endoscopy is currently less clear. In Korea, the NCSP for gastric cancer means that for patients electing to have upper endoscopy, Barrett esophagus can be effectively screened for at the same time with careful attention focused on the GEJ.
There is a consensus that once Barrett esophagus has been ruled out by a careful endoscopic examination, the chance of finding Barrett esophagus on a second follow-up gastroscopy is low, unless severe esophageal inflammation is present, which may mask underlying Barrett esophagus.
CONCLUSIONS
Increasing interest in identifying an effective strategy for decreasing the burden of EAC has been fuelled by the rising EAC rates worldwide, the morbidity associated with esophagectomy, and the development of endoscopic methods for curing early-stage EAC. In the face of this enthusiasm, however, we should be cautious about continuing our current evidence-free approach to screening and one with unclear benefits and unclear costs to the community.
In attempting to decrease the burden of EAC, perhaps more attention should be paid to primary prevention, namely risk factor modification such as minimizing obesity and smoking rates, although this has yet to be demonstrated. A better understanding of the risk factors for Barrett esophagus and progression to dysplasia and a more individualized risk calculation will be useful in defining populations to consider for Barrett screening. The development of novel, nonendoscopic screening techniques and of less expensive endoscopic techniques holds promise for a cost-effective screening and surveillance method to curtail the increasing rates of EAC.