Abdominal Compartment Syndrome in Severe Acute Pancreatitis Treated with Percutaneous Catheter Drainage
Article information
Abstract
Acute pancreatitis is one of the main causes of intra-abdominal hypertension (IAH). IAH contributes to multiple physiologic alterations and leads to the development of abdominal compartment syndrome (ACS) that induces multiorgan failure. We report a case of ACS in a patient with severe acute pancreatitis. A 44-year-old man who was admitted in a drunk state was found to have severe acute pancreatitis. During management with fluid resuscitation in an intensive care unit, drowsy mentality, respiratory acidosis, shock requiring inotropes, and oliguria developed in the patient, with his abdomen tensely distended. With a presumptive diagnosis of ACS, abdominal decompression through percutaneous catheter drainage was performed immediately. The intraperitoneal pressure measured with a drainage catheter was 31 mm Hg. After abdominal decompression, the multiorgan failure was reversed. We present a case of ACS managed with percutaneous catheter decompression.
INTRODUCTION
Abdominal compartment syndrome (ACS) is defined as a sustained intra-abdominal pressure (IAP) >20 mm Hg with evidence of organ dysfunction/failure.1 ACS may occur in association with medical or nonmedical critical diseases, and has been recognized as one of the causes of organ dysfunction.2 The risk factors for the development of intra-abdominal hypertension (IAH) or ACS are associated with diminished abdominal wall compliance, increased abdominal or intraluminal contents, capillary leakage, and excessive fluid resuscitation.3 Significant morbidity and mortality are associated with both IAH and ACS. Hence, screening for IAH/ACS risk factors in the intensive care unit (ICU) should be done, especially if complicated by new or progressive organ failure.3 A few cases of ACS associated with severe acute pancreatitis (SAP) have been reported since 2002.4 Patients with ACS had a significantly longer length of hospital stay, higher rates of systemic and local complications, and more invasive treatments.5
We report a case of ACS in a patient with SAP, who was treated with abdominal decompression by using percutaneous catheter drainage (PCD).
CASE REPORT
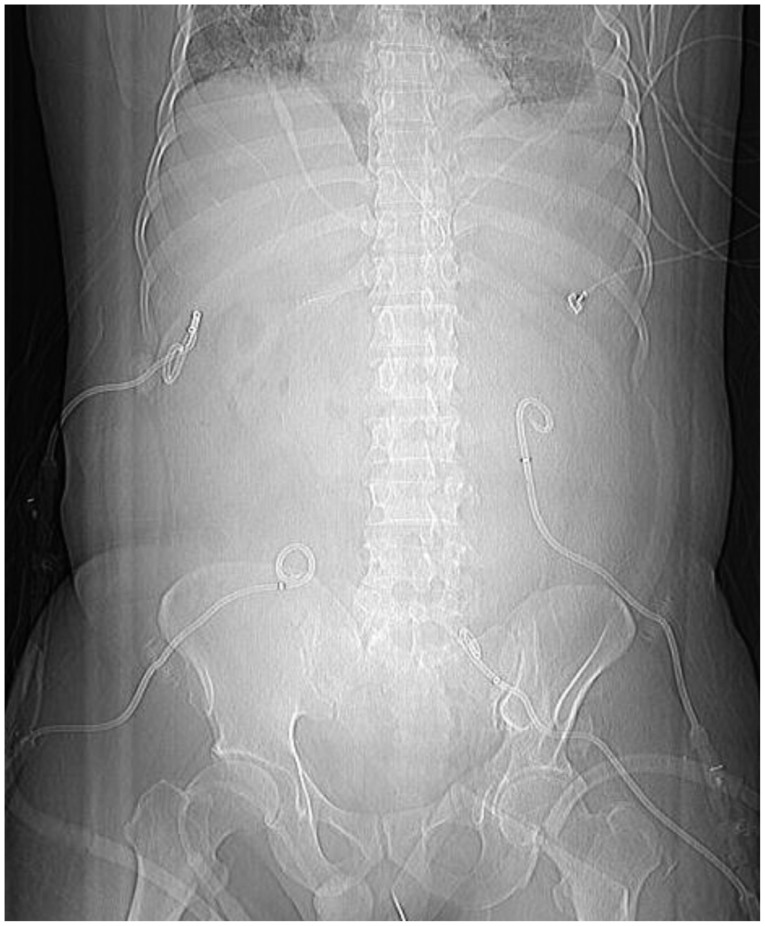
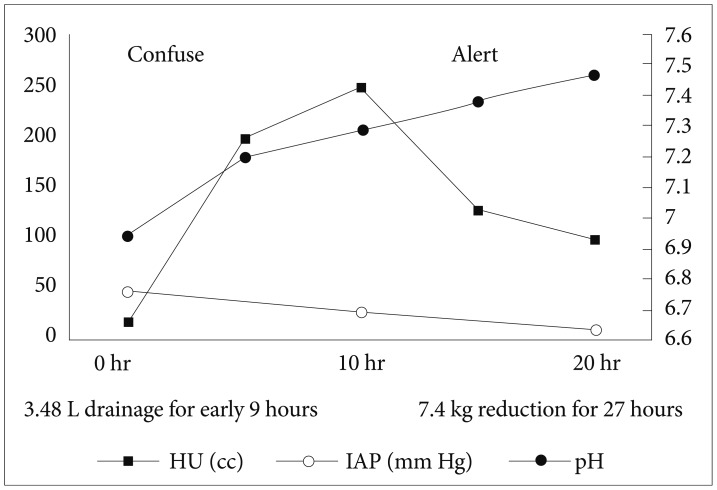
A 44-year-old man, who was previously healthy, was brought to the emergency department of our hospital, a tertiary referral center, in a drunk state. Initial physical examination revealed tenderness on the upper abdomen with normal bowel sounds. His vital signs were normal except for a mildly elevated body temperature of 37.8℃. Laboratory tests revealed the following: amylase 192 U/L (reference range, 13 to 53), lipase 948 U/L (reference range, 13 to 60), white blood cell count 24,900/mm3, hemoglobin 13.3 g/dL, hematocrit 40.1%, platelet count 304×103/mm3, aspartate aminotransferase (AST) 47 U/L, alanine aminotransferase (ALT) 24 U/L, lactate dehydrogenase 989 U/L, blood urea nitrogen (BUN) 14.2 mg/dL, creatinine 1.82 mg/dL, and high sensitivity C-reactive protein (hsCRP) 0.04 mg/dL. Abdominal computed tomography (CT) revealed mild swelling of the pancreas, peripancreatic fat infiltration, and fluid collection (Fig. 1A, B). Acute alcoholic pancreatitis was diagnosed, and the patient was managed with fluid resuscitation and antibiotics. On the third hospital day, he developed tachypnea, with 34 breaths per minute. His oxygen requirement was gradually increased to 10 L/min through a facial mask to maintain >90% oxygen saturation. He was transferred to the ICU, and acute respiratory distress syndrome (ARDS) was diagnosed (Fig. 2A). Mechanical ventilation, fluid resuscitation, and nasogastric tube drainage were initiated. On the 12th hospital day, his abdomen became tensely distended with no bowel sounds. His condition progressively deteriorated with the onset of drowsiness and the development of respiratory acidosis, shock requiring inotropes, and oliguria with a urine output of 40 mL/hr. Laboratory tests revealed the following: white blood cell count 23,200/mm3, hemoglobin 9.2 g/dL, hematocrit 28.2%, platelet count 336×103/mm3, AST 64 U/L, ALT 39 U/L, BUN 20.7 mg/dL, creatinine 0.80 mg/dL, and hsCRP 37.83 mg/dL. Arterial blood gas analysis showed the following: pH 6.96, pCO2 153 mm Hg, pO2 58.8 mm Hg, HCO3 32.5 mEq/L, and peak inspiratory pressure 67 mm Hg. Follow-up abdominal CT revealed a swollen pancreas with peripancreatic fluid collection, marked bowel edema, and ascites (Fig. 1C, D). The APACHE II score increased from 4 at ICU admission to 15 after 9 days, and the ARDS worsened (Fig. 2B). The possibility that IAH had progressed to ACS was assessed. To decompress the abdominal hypertension, 12-Fr pigtail drainage catheters (Cook, Bloomington, IN, USA) were inserted at four sites of the peritoneal cavity under ultrasonographic guidance at bedside (Fig. 3). Before drainage, intraperitoneal pressure (IPP) was measured by inserting a drainage catheter into the peritoneal cavity. The catheter was connected, through a fluid column, to an electronic pressure transducer with numeric and pressure trace displayed on the ICU monitor. IPP was measured in supine position with zero-reference level of the mid-axillary line. The mean of the values obtained at inspiration and expiration was 31 mm Hg. The drained fluid was compatible to pancreatic ascites showing exudates with dominant polymorphonucleocytes and elevated amylase level (621 U/L). After drainage of 3,480 mL fluid for 9 hours, the patient's IPP decreased to 19 mm Hg and his drowsiness, shock, respiratory acidosis, and oliguria resolved (Fig. 4). During the following 72 hours, he was managed with negative water balance and a body weight reduction of 7.4 kg was achieved. He remained stable with an IPP <12 mm Hg. He improved gradually, and weaning from a mechanical ventilator was done on the 15th hospital day (Fig. 2C). Follow-up abdominal CT showed decreased fluid collection at the peritoneum; however, colonic wall thickening remained (Fig. 1E, F). He was transferred to another referral center near his home on the 19th hospital day.
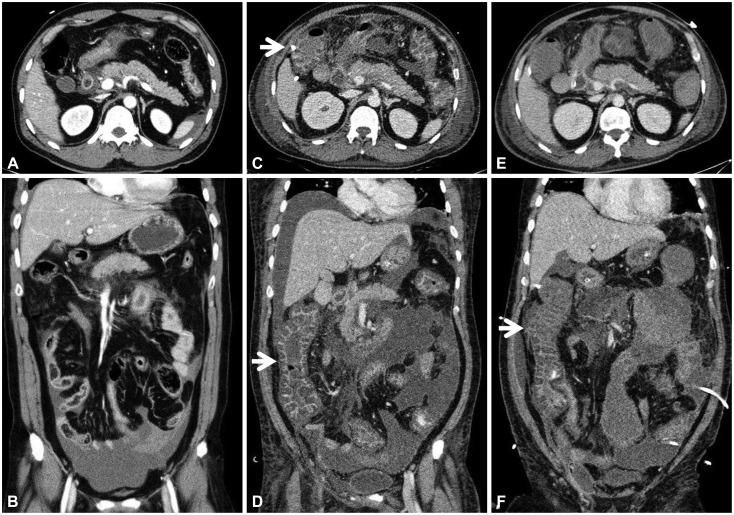
Abdominal computed tomography findings. (A, axial view; B, coronal view) Peripancreatic fat infiltration and fluid collection in the pelvic cavity were observed on admission. (C, axial view; D, coronal view) A swollen pancreas with peripancreatic fluid collection, marked bowel edema (arrows), and ascites were observed on the 12th hospital day. (E, axial view; F, coronal view) After abdominal decompression, fluid collection decreased in the peritoneum; however, colonic wall thickening (arrow) remained on the 17th hospital day.
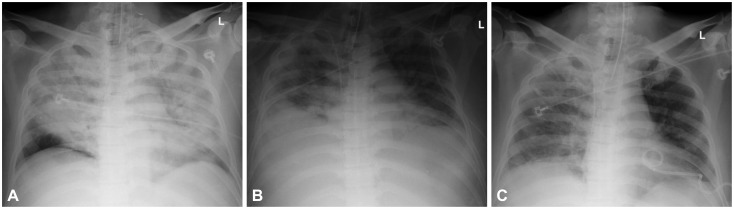
Chest radiograph findings. (A) Acute respiratory distress syndrome (ARDS) developed on the 3rd hospital day. (B) ARDS worsened on the 12th hospital day. (C) After abdominal decompression, ARDS improved on the 17th hospital day.
DISCUSSION
IAH and ACS have been increasingly recognized as causes of significant morbidity and mortality during the last decade. The World Society of the Abdominal Compartment Syndrome has recently developed consensus definitions outlining the standards for IAP measurement, IAH, and ACS.3 Elevated IAP induces splanchnic hypoperfusion, which decreases intestinal perfusion. Intestinal ischemia is believed to cause multiple organ dysfunction syndrome mediated by the inflammatory response. Sustained IAH can induce a significant dysfunction of cardiovascular, respiratory, renal, gastrointestinal, and central nervous systems.1
Organ failure, which results from SAP, is aggravated by IAH. In this case, the marked bowel edema with pancreatic necrosis observed in follow-up abdominal CT, oliguria, and respiratory failure suggested ACS. Through intensive abdominal decompression and fluid reduction of 3.5 L for 9 hours, the patient's clinical parameters improved, and he recovered.
Fluid resuscitation is the most important management step during the first 72 hours after the onset of SAP.6,7 Aggressive fluid therapy is recommended by current guidelines for acute pancreatitis, although there is no strong evidence to guide therapy.8,9 However, fluid overload is associated with complications such as acute lung edema, cerebral edema, IAH/ACS, and extensive soft tissue edema. This is especially true in patients who have developed cardiovascular dysfunction or a pulmonary capillary leak syndrome.7 In our case, aggressive fluid replacement in the setting of increased capillary permeability resulted in complications such as bowel ischemia, edema, IAH, and respiratory acidosis. After the initial 24 hours of care, a strategy of increasing the fluid volume and infusion rate, tailored to the individual patient's specific clinical and objective findings, should be used. Which fluids are optimal and should be provided to the patient is still unclear. A recent study showed that fluid resuscitation with hydroxyethyl starch in the early stages of SAP can decrease the risk of IAH and reduce the use of mechanical ventilation.10
Early surgical intervention in patients with SAP is not currently recommended, and conservative treatment is the gold standard. The condition of ACS in SAP has attracted increasing attention recently. Patients with this condition are candidates for intervention. Surgical decompression may be the optimal solution; however, mortality still remains high, and it is a labor-intensive procedure accompanied by an increased risk of enteric fistulas, hemorrhage, and possibly a greater incidence of infection and necrosis.11 PCD reduces surgical intervention but it might not be sufficient in very severe patients. Some literature reports suggest that PCD should be the first step in the treatment of patients with IAH/ACS. Only patients who do not respond to this type of treatment should be considered candidates for surgical decompression.12
Because the complications of an increase in IAP are life-threatening, methods to correctly measure pressure in the abdominal cavity were developed. Various techniques, such as intragastric, intracolonic, intravesical, and intravenous techniques, have been used to measure IAP indirectly.13 On the other hand, IAP is directly measured by using an intraperitoneal catheter connected to a pressure transducer.14 Among the various methods, intravesicular pressure (IVP) is the gold standard in detecting elevated IAP. It should be measured with a catheter inserted into the bladder, at end expiration, with the patient in the supine position and zeroed at the level of the mid-axillary line after the bladder is fully emptied and then filled with 25 mL saline.13 In this case, IAP was checked by using a peritoneal catheter, not with IVP, because the patient's condition was rapidly deteriorating, requiring prompt decompression with PCD. A recent study showed that IPP was not different to IAP in human15 and animal models.16 The authors compared the IPP measured by using a peritoneal dwelling catheter to the IAP measured by using an intravesicular catheter in 25 patients treated with peritoneal dialysis.15
We reported a case of ACS caused by fluid resuscitation in the setting of acute severe pancreatitis with recovery through PCD. In SAP, IAH/ACS is a factor indicating a poor prognosis. If the patient has risk factors for IAH/ACS, frequent measurement of IAP is recommended.17
Notes
The authors have no financial conflicts of interest.