INTRODUCTION
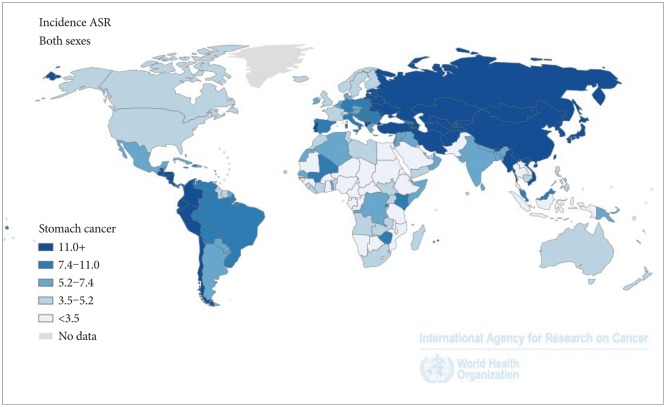
In spite of declining incidence in recent years, gastric cancer is still one of the most frequently occurring cancers. The most recent estimates from the International Agency for Research on Cancer (IARC) GLOBOCAN 2012 indicate that nearly one million new gastric cancer cases and more than 700,000 deaths occurred globally in 2012.1 The disease accounted for 7% of the total new cancer cases and 9% of the total cancer deaths. Gastric cancer is now the fifth most common cancer in the world, after lung, breast, colorectal and prostate cancer and the third leading cause of cancer death.1 There is a 10-fold variation in gastric cancer incidence internationally, with high rates seen in many countries of Eastern Asia (age standardized rate, 42.4/100,000 in 2008), Central and Eastern Europe (21.9/100,000), and Central (12.7/100,000) and South America (17.3/100,000), and much lower rates reported from North America (5.8/100,000), and Africa (4.7/100,000) (Fig. 1). In addition, rates in men are approximately double those observed in women.1,2
Over the past four decades, the age-standardized incidence as well as mortality rates from gastric cancer have steadily declined in nearly all populations, independently from their background risk of gastric cancer or sex (Fig. 2).3 Allowing for the estimated annual percentage change of -2%, the annual number of new gastric cancer cases (both sexes) would still slightly increase or remain stable in the year 2030 due to ageing of the population.1,4 The relative 5-year survival rates for gastric cancer are low in most countries with less than 30% except for the Republic of Korea and Japan with around 70% where screening programmes that lead to early detection are in place. The case fatality rate is lower in countries with high levels of human development (overall mortality-to-incidence ratio=0.65) than in countries at low or medium levels of human development (0.83).2
Currently, the incidence rates of gastric cancer among men in the Republic of Korea is the highest in the world.4 It has been estimated that the age-standardized rates were 63.3 in men and 25.1 per 100,000 in women, respectively, with approximately 32,000 new gastric cases being diagnosed in 2011.5 The rates have remained stable for many years (67.2 in 2001 and 63.3 in 2011 for men; 26.2 in 2001 and 25.1 in 2011 for women) without geographical variation across the country.6 Gastric cancer was the most frequently occurring cancer in men, accounting for 19.6% of all cases and the third most frequently diagnosed cancer in women, accounting for 10.3% of all cases occurred in Koreans in 2012.7 Recent mortality data in Korea showed that gastric cancer was the third leading cause of cancer death in men, and the second leading cause of cancer death in women.5 The mortality rate for gastric cancer has decreased continuously both in men and women since 1997. The 5-year survival rate for gastric cancer in Korea have been much improved over the last two decades, showing an increase from 43% in 1993 to 1995 to 67% in 2006 to 2010.7
GASTRIC CANCER TYPES AND CLASSIFICATIONS
Gastric cancer represents a biologically and genetically heterogeneous group of tumours with multifactorial aetiologies, both environmental and genetic.2,8 Gastric cancers arising from the most proximal (cardia) or distal (non-cardia) region are likely to have different aetiologies. The majority of gastric cancers are noncardia cancers, while cardia cancers have been most commonly reported in North American and European populations.2
In most cases of noncardia gastric cancer, the cancer develops from the epithelial cells of the gastric mucosa with a transition from dysplasia to confirmed cancer. The successive steps in the sequence of gastric carcinogenesis, in relation to Helicobacter pylori infection of the mucosa, have been described by Correa.9 Those steps include: (1) chronic atrophic gastritis of the mucosa up to the lamina propria by the inflammation preceded by H. pylori bacterial infection; (2) intestinal metaplasia in large areas of the epithelium; (3) premalignant adenomatous lesion with succession of low and high grade dysplasia; and (4) intramucosal cancer.
Histological classification of gastric cancer
One of the most commonly used histological classifications of gastric cancer is the Laur├®n classification, where diffuse and intestinal types are identified as the two major subtypes.10 Mixed and indeterminate types are relatively uncommon.10 Diffuse type consists of poorly cohesive cells with little or no gland formation while intestinal type forms glands with various degrees of differentiation.8
The World Health Organization (WHO) classification is another widely used classification and it recognizes five major types: (1) tubular; (2) papillary; (3) mucinous; (4) poorly cohesive, including signet ring cell carcinoma and other variants; and (5) mixed carcinomas. This is a descriptive scheme and does not take into account histogenesis, differentiation or epidemiological data.8 Tubular and papillary types in the WHO classification roughly correspond to the intestinal type in the Laur├®n classification, and poorly cohesive cancers correspond to the diffuse type.2
Classification of dysplasia/intraepithelial neoplasia
The well-known discrepancies between Japanese and European/North American pathologists in categorizing gastric dysplasia/intraepithelial neoplasia11,12 resulted in attempts to standardize the terminology of the morphological spectrum of lesions ranging from non-neoplastic changes to early invasive cancer.8,13 The Vienna classification of gastrointestinal epithelial neoplasia is one of them and the categories are designed to be associated with different recommendations for clinical management. The Vienna Classification has been revised and it identifies five categories: (1) negative for neoplasia/dysplasia; (2) indefinite for neoplasia/dysplasia; (3) mucosal low grade neoplasia (low grade adenoma; low grade dysplasia); (4) mucosal high grade neoplasia which is subdivided into: 4.1) high grade adenoma/dysplasia; 4.2) noninvasive carcinoma (carcinoma in situ); 4.3) suspicious for invasive carcinoma; 4.4) intramucosal carcinoma; and (5) submucosal invasion by carcinoma. This revised Vienna classification aims to be more closely related to patient management, with more clinical usefulness.12
For premalignant lesions, the current 2010 WHO classification considers: (1) negative for intraepithelial neoplasia (dysplasia); (2) indefinite for intraepithelial neoplasia (dysplasia); (3) intraepithelial neoplasia (dysplasia) which is further subdivided into two grades: 3.1) low-grade intraepithelial neoplasia (dysplasia) and 3.2) high-grade intraepithelial neoplasia (dysplasia); and (4) intramucosal invasive neoplasia/intramucosal carcinoma.8 The WHO classification acknowledges that both dysplasia and intraepithelial neoplasia are acceptable terms.
Despite these attempts to lessen disagreements between Japanese and European/North American, lesions diagnosed as intramucosal carcinoma in Japan are still classified as high-grade adenoma/dysplasia in Europe/North America and often not diagnosed as cancer.14 In many cases neither Western nor Japanese pathologists could distinguish between "high grade adenoma/dysplasia," "noninvasive carcinoma (carcinoma in situ)," and "suspicion of invasive carcinoma" in a reproducible way.15
Endoscopic classification
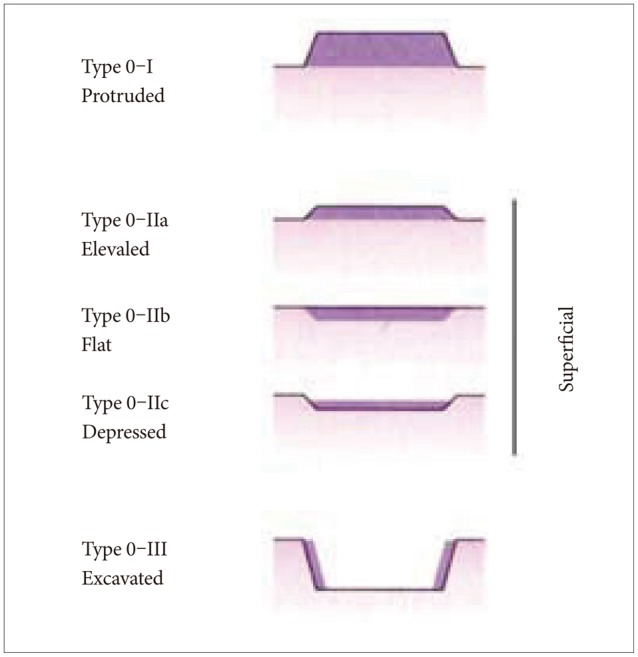
Gastric cancers are classified endoscopically according to growth pattern.8 Early gastric cancer is an invasive carcinoma that is limited to the mucosa, or the mucosa and submucosa, regardless of nodal status. Early gastric cancers are divided into three types (Fig. 3): (1) protruded (type 0-I); (2) superficial (type 0-II); and (3) excavated (type 0-III). The superficial type accounts for the majority of early gastric cancers and is further divided into type 0-IIa (elevated type), type 0-IIb (flat type), and type 0-IIc (depressed type).8,14
The Paris endoscopic classification of superficial neoplastic lesions of the stomach defines "superficial" at endoscopy when the endoscopic appearance suggests that the depth of penetration in the digestive wall is not more than into the submucosa, i.e., there is no infiltration of the muscularis propria.16 This classification considers the tumour grossly and endoscopically.17
In contrast to early gastric cancer, the gross appearance of advanced carcinoma is described macroscopically according to the Borrmann classification which includes: (1) polypoid type; (2) fungating type; (3) ulcerated type; and (4) infiltrative type, in which fungating and ulcerated types are common.8 With advanced gastric cancer invading to the proper muscle layer or beyond, 5-year survival rate was around 60% or less18 while early gastric carcinoma has a favourable prognosis, with a 5-year survival rate as high as 90%19,20 or higher even in elderly patients.21 A recent study of 598 Korean patients and 159 United States patients showed 5-year probability of death due to early-stage node-negative (T1N0) gastric cancer was 1.6 % in Korean patients and 3.2% in the United States patients between 1995 to 2005.22
Precursor lesions: gastritis
One of the recognized precursor lesions of the intestinal type, distal gastric cancer is gastritis. Despite the general consensus on the morphological aspect of the lesions, there have been controversies over the different types and pattern of gastritis, which led to considerable confusion among pathologists.23 In an attempt to establish an agreed terminology of gastritis and to identify, define and resolve some of the problems associated with the Sydney System, the Updated Sydney System was proposed as one of the most representative histological methods for gastritis assessment. The Updated Sydney system recommends an itemized description of various microscopic findings including lymphoplasma cell infiltrates, neutrophilic infiltrates, intestinal metaplasia, mucosal atrophy, and H. pylori. Severity/intensity of each factor is quantitatively expressed using a four-point grading system of the histological lesions, ranging from 0 (absence) to 3 (marked).23
Although the Updated Sydney System has been widely used with useful recommendations, it does not provide a clinically meaningful link between the presence of gastritis and risk of developing gastric cancer with no immediate prognostic or therapeutic information to clinicians.24 Recognizing the consistent evidence of the association of the extent of atrophy with the risk of gastric cancer, the Operative Link for Gastritis Assessment (OLGA) staging system was proposed.24 The OLGA staging system ranks gastritis-associated cancer risk according to both the topography and the extent of gastric mucosal atrophy.25 It stages corpus and antrum from stage 0 (no atrophy) to stage IV (severe atrophy) where the risk to develop gastric cancer is substantially high.24 A modification of the OLGA staging system, the Operative Link on Gastric Intestinal Metaplasia (OLGIM) assessment replaces the atrophy score with an assessment of intestinal metaplasia alone but is still based on the OLGA frame.26 Since the OLGIM proposal replaces the global atrophy score with a semiquantitative assessment of intestinal metaplasia both for extent and site, it has a higher inter-observer agreement compared with the histopathological diagnosis of atrophic gastritis.26 However, OLGIM staging alone is less sensitive than OLGA staging in the identification of patients at high risk of gastric cancer because it primarily focuses on intestinal metaplasia.26 Nonetheless, both OLGA and OLGIM gastritis staging systems have contributed to provide prognostically important information on the gastritis-associated cancer risk.
HEREDITARY SYNDROMES AND GENETIC PREDISPOSITION
Only a very small proportion of gastric cancer cases is linked to germline mutation in E-cadherin/CDH1, which has been found in approximately 25% to 40% of families with hereditary diffuse gastric cancer.27,28 The penetrance of CDH1 gene mutations is high, with an estimated risk of >80% by the age of 80 years with poor prognosis and late presentation with a highly invasive diffuse type tumour.29
Two major autosomal dominant forms of heritable colorectal cancer, familial adenomatous polyposis and hereditary nonpolyposis colorectal cancer (Lynch syndrome), are also linked to increased risk of other cancers including gastric cancer. Lynch syndrome is caused by germline mutations in four mismatch repair genes (MLH1, MSH2, MSH6, and PMS2), resulting in development of a spectrum of different tumours and Lynch syndrome mutation carriers have shown a substantial risk for developing gastric cancer.30 The risk of gastric cancer is also increased in Li-Fraumeni syndrome with germline mutation of TP53.31
Peutz-Jeghers syndrome is an inherited multiple gastrointestinal polyposis associated with mucocutaneous pigmentation.32 Germline mutation in the LKB1 gene that encodes serine/threonine-protein kinase that acts as a tumour suppressor results in the loss of LKB1 function which can lead to gastric cancer carcinogenesis.33
While hereditary gastric cancer is uncommon, genetic alterations in sporadic gastric cancer cases are frequently reported. However, no major high-penetrance genes have been discovered so far,29 although genetic factors might play an important role in gastric carcinogenesis by possibly influencing immune and inflammatory responses especially to H. pylori infection and thereby altering susceptibility to gastric cancer.34
Polymorphisms of the interleukin 1╬▓ gene (IL-1╬▓), which contributes to initiation and amplification of inflammatory response, and of the interleukin-1 receptor antagonist (IL-1RN) gene, which modulates inflammation, have been associated with gastric cancer risk. However, a systematic review of 35 studies did not find an overall association of polymorphisms in these inflammatory genes with risk of gastric cancer, but there was significant heterogeneity among study results.35 Results from very large genome-wide association studies (GWAS) showed reproducible associations between single-nucleotide polymorphisms located at prostate stem cell antigen gene (PSCA), PLCE1, and Mucin 1, cell surface-associated gene (MUC1) genes and different subtypes of gastric cancer risk. These results from GWAS are mainly from Chinese, Korean and Japanese populations and the clear biological mechanism behind these polymorphisms is not yet clearly known.29
RISK FACTORS FOR GASTRIC CANCER
The main cause of gastric cancer is chronic infection with H. pylori.36,37 The IARC classified H. pylori as a class I carcinogen in 1994 and reconfirmed this classification in 2009 after reviewing newer evidence.38 According to the most recent estimates using a more sensitive method to detect H. pylori, approximately 89% (770,000 cases) of distal gastric cancer cases worldwide have been estimated to be attributable to chronic H. pylori infection.39
H. pylori infection is present throughout the world and causes most cases of peptic ulcer disease and mucosa-associated lymphoid tissue lymphoma, as well as gastric cancer. The prevalence of infection in adults varies between 20% to 50% in industrialized countries and over 80% in many developing countries.40
H. pylori infection is strongly associated with socioeconomic conditions,41 and transmission appears to occur through close personal contact, particularly within the family38 and typically in early childhood. Once established, infection usually persists throughout life unless treated. The prevalence of H. pylori infection has decreased in recent decades, particularly among children in developed countries, probably reflecting improvements in hygiene.42,43 However, there is only limited information on prevalence trends among children from most countries,41,44 and H. pylori still infects an estimated 50% of the world's population.
Factors associated with colonization and pathogenicity of H. pylori include virulence factors like cytotoxin-associated gene A (cagA) in the cag pathogenicity island and the vacuolating cytotoxin A (vacA). cagA is a gene that is part of the cag pathogenicity island and colonization with strains that possess cagA is associated with increased risk of developing both intense tissue responses and premalignant and malignant lesions in the distal stomach through secreting a functional cytotoxin which induces more severe gastric injury and further augment the risk.45,46 CagA has been shown to associate with the apoptosis-stimulating protein of p53, inhibiting the apoptotic function of p53, in a manner similar to human papillomavirus (HPV) and other oncogenic DNA viruses.47
vacA encodes a secreted bacterial toxin (VacA) and induces multiple structural and functional alterations in cells, the most prominent of which is the formation of large intracellular vacuoles.48 Primarily due to variations in vacA gene structure, vacA positive strains vary considerably in production of cytotoxin activity, despite its universal presence in all H. pylori strains examined.45
Another infectious agent that has been associated with gastric cancer is the Epstein-Barr virus (EBV). The virus is a ubiquitous infectious agent with a prevalence of over 90% in adults and has been causally linked to the development of several malignancies, including Burkitt's lymphoma, immunosuppression-related lymphoma, Hodgkin's disease, and nasopharyngeal carcinoma.49 Approximately 8% of gastric cancers have been estimated to harbour EBV,50 but there is insufficient epidemiological evidence of a clear etiological role for EBV in gastric carcinogenesis.38 The EBV genome is present in the tumour cell in a monoclonal form, and transforming EBV proteins are expressed in the tumour cell. A meta-analysis reported that the prevalence of EBV-positivity in gastric cancer varied jointly by age, sex and anatomic subsite, i.e., EBV-positivity decreased with age among men, more steeply for tumours localised to the antrum.50 In addition, EBV-positivity has been associated with longer survival,51 suggesting that EBV-positive gastric cancer may have distinct clinical and genetic features, and therefore may be a separate clinical entity.
Various other risk factors including older age, male sex, cigarette smoking, low socioeconomic status, low level of physical activity, and radiation exposure have been linked to increased risk of gastric cancer in general while gastroesophageal reflux disease and obesity have been associated with increased risk of gastric cardia cancer specifically.29 Nonsteroidal anti-inflammatory drugs and statin intake have shown inverse associations with gastric cancer risk.29
Dietary factors have been investigated in many studies in relation to gastric cancer risk, since food carcinogens can potentially interact directly with the epithelial cells that line the stomach. A comprehensive systematic review of dietary factors and cancer prevention published by the World Cancer Research Fund/American Institute for Cancer Research (WCRF/AICR) concluded that nonstarchy vegetables, including specifically allium vegetables, as well as fruits probably protect against gastric cancer while salt, and also salt-preserved foods, are probably causes of this cancer.52 A high concentration of sodium chloride has been shown to cause damage to the gastric mucosa, cell death and consequent regenerative cell proliferation in animal models,53,54 which may lead to inflammation and damage such as diffuse erosion and degeneration.55 Studies have found that a high salt diet was associated with colonization of H. pylori56 and exacerbated H. pylori-induced inflammation,57 which in consequence was responsible for promotion of gastric carcinogenesis in a dose-dependent fashion.58 This was also confirmed in other studies showing that salt worked synergistically with H. pylori infection to enhance the expression of proinflammatory enzymes and cytokines such as inducible nitric oxide synthase and cyclooxygenase-2 in the gastric mucosa.59 In addition, high salt concentrations have been shown to stimulate increased expression of H. pylori CagA, which in turn leads to an increased amount of CagA translocated into gastric epithelial cells and an enhanced ability of H. pylori to alter gastric epithelial cell function.60
Another dietary factor that might play a role in gastric carcinogenesis is N-nitroso compounds.52 Nitrates, produced endogenously in gastric acid, and added as preservatives to processed meats may contribute to N-nitroso compound production and exposure that are suspected mutagens and carcinogens.52 Previous studies have shown a significant increase of gastrointestinal cancer risk associated with dietary61 or endogenous exposure to N-nitroso compounds,62 especially with noncardia gastric cancer rather than cardia gastric cancer.62 Many processed meats contain high levels of salt and nitrite. Haem found in red meat promotes the formation of N-nitroso compounds through direct reaction between nitric oxide and haemoglobin and myoglobin.61 Red meat also contains iron, which can lead to the production of free radicals. Vitamin C may be protective against H. pylori-associated gastric carcinogenesis by enhancing mucosal immune response, neutralizing free radicals, reducing the formation of gastric N-nitroso compounds, inhibiting cell proliferation and directly affecting H. pylori growth.62 The gastric juice vitamin C levels were also shown to be lower with infection with CagA positive H. pylori strains compared with CagA negative H. pylori strains.63,64 Even with the biologically plausible mechanisms, epidemiological evidence suggesting that dietary nitrate and nitrite or other N-nitroso compounds are the causes of gastric cancer is limited. The WCRF/AICR report assessed other dietary factors and concluded that the available evidence suggesting that pulses (legumes) and foods containing selenium protect against gastric cancer and that chilli, processed meat, smoked foods, and grilled (broiled) and barbecued (charbroiled) animal foods are causes of stomach cancer is also limited.52
PRIMARY PREVENTION: EFFECTIVENESS OF H. pylori ERADICATION IN GASTRIC CANCER PREVENTION
The two major primary prevention strategies for gastric cancer at a population level might include improvement in diet and reduction in the prevalence of H. pylori, the main cause of gastric cancer.2 Prevention through dietary intervention would be with increased fruit and vegetable intakes and decreased consumption of salt or salt-preserved foods. Lifestyle modification such as increased physical activity and smoking cessation may also help reduce risk of getting the disease. Interventions for primary prevention at the population level may potentially involve very large numbers of people that require convincing evidence of effectiveness. However, interventions based on these dietary strategies have not yet been evaluated in large-scale randomized clinical trials examining their impact on gastric cancer incidence or mortality.
Another strategy to prevent gastric cancer would be through H. pylori eradication. Recognition of the causal role of chronic H. pylori infection in gastric cancer led some authors by 2005 to call for broad-scale programmes to eradicate the infection as a way to prevent the disease.65 However, no countries with high burden of gastric cancer have yet implemented a population-based H. pylori eradication programme. Some of this inaction may reflect doubts about the effectiveness of H. pylori eradication in preventing gastric cancer as there are still scant data available from randomized clinical trials.66,67 Recently, a new meta-analysis of all six reported randomized trials among asymptomatic adults estimated a benefit (relative risk=0.66, 95% confidence interval [CI], 0.46 to 0.95).68 In 2012, a trial in a general population of adults in China reported a statistically significant reduction in gastric cancer risk following treatment after 15 years of follow-up (4.6% in control group, 3.0% in treated group; odds ratio, 0.61; 95% CI, 0.38 to 0.96).69 Two trials have assessed the effect of H. pylori treatment after endoscopic mucosal resection of early gastric cancer.70,71 One of the trials, in Japan, reported a statistically significant reduction in risk of metachronous gastric cancer.71 Risk also appeared lower in a study conducted in the Republic of Korea, but the result was not statistically significant.70 Although these results of randomized trials indicate that H. pylori treatment may lower gastric cancer incidence by 30% to 40%, there are important limitations to the available data. Firstly, the meta-analysis finding is based on relatively few trials, many of which had too few cancer endpoints to contribute much weight to the analyses. Secondly, because of the relative paucity of endpoint data, the estimated size of the overall beneficial effect of treatment is likely to be imprecise. Thirdly, the larger trials were all conducted among middle-aged adults in Asia, notably in China, and the generalizability of their results to other populations is not certain. Lastly, the trials do not provide direct data on the feasibility, costs, effectiveness, and adverse effects of treatment programmes as they are applied in community settings.72
A recently published report of a working group of the IARC of the WHO concluded that-randomized clinical trials have found that H. pylori treatment is effective in preventing gastric cancer, and models indicate that H. pylori screening and treatment strategies would be cost-effective. However, uncertainties remain about the generalizability of results and about the cost-effectiveness and possible adverse consequences of programmes applied in community settings. The Working Group therefore recommends that countries explore the possibility of introducing population-based H. pylori screening and treatment programmes, but cautions that decisions as to whether and how to implement H. pylori testing and treatment must hinge on local considerations of disease burden, other health priorities, and cost-effectiveness analyses. Moreover, these programmes should be implemented in conjunction with a scientifically valid assessment of programme processes, feasibility, effectiveness, and possible adverse consequences."73,74
SECONDARY PREVENTION: SCREENING
Early detection efforts
Early detection and treatment of cancer requires accessible, high-quality health services with adequate human, financial, and technical resources. Screening methods that are based on rigorous evidence and are implemented in national or regional cancer screening programmes currently include: mammography for breast cancer; cytology, HPV testing and visual inspection of the cervix for cervical cancer; and faecal occult blood testing or faecal immunochemical testing, flexible sigmoidoscopy, and colonoscopy for colorectal cancer.2 With the exception of the republic of Korea (see below), gastric cancer screening is generally not included in national cancer prevention strategies, even in countries with a high burden of the disease. This may reflect not only high cost, decreasing incidence worldwide and the paucity of data to answer the questions, "how, who, and when to screen." It may also result from the complexity of the screening process and the substantial knowledge, skill, and effort needed to assure the quality of the screening services.2,75 Given the wide variation in gastric cancer burden around the world and the substantial numbers of cases and deaths projected in the coming years, further improvement in early diagnosis and treatment, and development and validation of effective methods for population-based screening in countries with a significant projected burden of disease should be a priority on the public health agenda.
Different types of tests have been proposed and used in a few countries for gastric cancer screening. Barium-meal photofluorography (or barium swallow) has been used for mass screening in Japan since 196076 and upper endoscopy has been used for nationally organized gastric cancer screening in the Republic of Korea since 1999.77 Active intervention in a population usually requires randomized controlled clinical trial data showing that the intervention is effective.2 Photofluorography has been used for screening in the absence of evidence for reducing gastric cancer mortality in randomized controlled clinical trials.75 Current guidelines for gastric cancer screening in Japan that recommended use of photofluorography for both population-based and opportunistic screening, are largely based on results of case-control studies which suggested a 40% to 60% decrease in gastric cancer mortality with photofluorography.78 Prospective cohort studies were limited in numbers and inconsistent with the results in demonstrating the reduction in gastric cancer mortality. The sensitivity of photofluorography ranged from 60% to 80%, whereas the specificity ranged from 80% to 90%.78
Upper gastrointestinal endoscopy has been regarded as the gold standard for the diagnosis of gastric cancer.29 It is also used for minimally invasive treatment of early gastric cancer through endoscopic mucosal resection and endoscopic submucosal dissection. Widespread use of the latter method has resulted in a dramatic increase in the number of early gastric cancer cases treated endoscopically in Japan and Korea.14 In high risk populations it may also play a role in secondary prevention (screening) as the examination used for primary testing for gastric cancer or its precursors. The technique is highly dependent on the skills of the endoscopists; however, and the feasibility of endoscopy-based screening depends on capacity that is limited in most countries. Endoscopic screening was shown to be cost-effective in areas with high gastric cancer burden,79 although further studies are needed to determine the suitability of this technique for recommending its broad use as a primary screening test.80
The effectiveness of endoscopic screening to reduce gastric cancer mortality has not yet been confirmed in randomized controlled trials. A study conducted retrospectively in a community in an isolated island in Japan found that in the district where endoscopic screening was introduced, the odds ratio of death from gastric cancer among the participants versus non-participants of endoscopic screening was 0.12 (1 case out of 16 screened vs. 8 cases out of 22 nonscreened), while it was 0.09 (2 out of 34 screened vs. 48 out of 114 nonscreened) in the radiographic screening district, after 6 years of follow-up on average, suggesting that both radiographic and endoscopic screening may prevent death from gastric cancer.81 However, very small numbers analysed in the study and its retrospective nature limit interpretation of the study.
In a cohort study carried out in Linqu County, China with a high incidence of gastric cancer, the number of observed deaths from gastric cancer (n=37) during 11 years of follow-up with repeated endoscopic screening was close to the number expected (n=36.8), resulting in a standardized mortality ratio of 1.01 (95% CI, 0.72 to 1.37) for men and women combined.82 The most recent evidence comes from a community-based case-control study conducted in Japan, which suggested a 30% reduction in gastric cancer mortality by endoscopic screening compared with no screening within 36 months before the date of diagnosis of gastric cancer.83 The study results must be interpreted cautiously; however, due to various methodological limitations. For example, symptomatic individuals were not excluded and no other background information was collected which might have explained the results.
In Korea, the performance of upper endoscopy has been intensively studied within the setting of the National Cancer Screening Programme (NCSP) especially in comparison with upper gastrointestinal series (UGIS), which is another modality that participants can choose for gastric cancer screening. The detection rates of gastric cancer were 0.68 and 2.61 per 1,000 UGIS and endoscopy screenings, respectively.80 The sensitivities of UGIS and endoscopy screening to detect gastric cancer were 36.7% and 69.0%, respectively, and their specificities were 96.1% and 96.0%, respectively.80 The detection rate of gastric cancer by endoscopic screening is 2.7- to 4.6-fold higher than that by gastric radiography.80,84,85 Overall, endoscopy performed better than UGIS in the NCSP for gastric cancer. The unit costs of screening using UGIS and upper endoscopy were US $32.67 and $34.89, respectively. In 2008, the estimated cost of identifying one case of gastric cancer was US $53,094 using UGIS and $16,900 using upper endoscopy.84 In a study of more than 18,000 participants who underwent endoscopy within NCSP between 2001 and 2007, a total of 81 gastric cancer cases were found, in which 81% were early gastric cancers.86 The study reported a 50% decreased incidence of gastric cancer in the repeated endoscopic screening group within 2 years compared the group with infrequent screening. In addition, gastric cancers detected by a repeated endoscopy within 2 years were found to be mostly early gastric cancers that can be treated by endoscopic resection.86
Pepsinogen for atrophic gastritis detection
Plasma biomarkers tests may identify patients at increased risk of developing gastric cancer, especially with atrophic gastritis, who would then be eligible for endoscopic surveillance for cancer risk.87 The method that has been investigated most extensively is pepsinogen levels.
Pepsinogens I and II are produced by cells from the gastric mucosa, whose production can be altered by atrophic gastritis.87 These pepsinogen alterations have been shown to be a marker for atrophy in the gastric mucosa. Since atrophic gastritis is the highest known independent risk factor for distal, noncardia gastric cancer,87 the test for pepsinogen levels has long been considered for screening to identify a high risk group. A pooled analysis of Japanese studies in a total of about 300,000 individuals, pepsinogen test resulted in a sensitivity of 77% and a specificity of 73% for atrophic gastritis, suggesting the test is reliable as a marker for the identification of individuals at risk of progression to intestinal gastric cancer.88
Both Chinese and Japanese studies89,90,91 have shown a synergistic effect of both H. pylori and pepsinogens, i.e., the highest risk of developing gastric cancer shown in the group positive for pepsinogen test but negative for H. pylori, suggesting that the utility of the pepsinogen test might be improved if the test is combined with serology for H. pylori infection. Outside Asia, however, evidence for the utility of pepsinogen test has been less convincing, especially in Latin America, requiring further studies to establish the performance of the test and define cutoff values for the population.92 Moreover, it should be noted that the pepsinogen test is not useful for screening of diffuse gastric cancer, and performance would vary depending on the proportion of diffuse gastric cancer in the population.92 Considering that the pepsinogen test is still too expensive to be applied in community-based screening programmes, particularly in low-middle income countries in Latin America and Asia that present the highest mortality rates, the possible population-based application needs to be further evaluated.92
More recently, amidated gastrin-17 (G-17) has been suggested to characterize atrophy in the antral part of the stomach. G-17 is secreted exclusively by the G cells in antrum, and G-17 levels have been evaluated as a serological marker to distinguish antral atrophic gastritis from corpus atrophic gastritis. In particular, patients with antral atrophic gastritis present with low levels of circulating G-17.87,93,94 However, the concentrations of G-17 in the circulation are influenced by several factors, and as a result the sensitivity for diagnosing atrophy in the antral part of the stomach is not satisfactory (15.4% in a fasting state and 30.8% after stimulation),95 possibly due also to inadequate performance of available commercial kits.92 In a recent study that evaluated associations of temporal changes in multiple serological markers including gastrin-17 with risk for progression of gastric precancerous lesions during 14 years of follow-up, changes in gastrin-17 were not statistically significantly associated with progression.96 Given that there are very few epidemiological studies of G-17 in relation to gastric cancer, the value of G-17 for gastric cancer screening is unclear.
CONCLUSIONS
Gastric cancer remains the third leading cause of cancer death worldwide and the burden of disease, which for unknown reasons affects twice more men than women and varies widely from place to place, is expected to remain high for many decades despite declining trends. The disease is usually detected late and survival is poor in most settings. With few exceptions, there are no public health programmes aimed at reducing gastric cancer burden.
Primary prevention interventions, including dietary modifications and eradication of H. pylori need to be considered as potentially valuable tools in the control of this disease. H. pylori can be treated with antibiotics and in clinical trials, its eradication has been shown to reduce gastric cancer incidence between 30% and 40%. However, given the high prevalence of infection in high risk areas and limited information about feasibility, acceptability and potential adverse consequences of the intervention, it has not been broadly implemented. Several trials are underway that should provide additional data, but demonstrations projects with proper evaluation of the programme were recently recommended by an IARC working group of international experts.
More rapid impact on the burden of disease might be forthcoming from secondary prevention through early detection and prompt treatment of precursor lesions and early cancer. Encouraging progress has been achieved in Japan where opportunistic screening is widespread, and in the Republic of Korea where screening is conducted in an organized programme. However, the methods used in these countries require infrastructure and human resources with ample expertise which are not available in less developed areas. In addition, the effectiveness of the screening methods has not been validated in randomized controlled clinical trials.
Endoscopic detection and treatment is only one important facet of the complex process of gastric cancer screening. To achieve an appropriate benefit, screening should only be considered in regions with a significant burden of disease.97,98,99 In addition, screening should be conducted in organized, population-based programmes to promote equal access through personal invitation and to facilitate comprehensive quality assurance systems. Before gastric cancer screening can be recommended as a public health policy in other countries, information is required on expected detection rates, participation rates, acceptability, economic costs, as well as any complications or adverse effects associated with screening in different populations. The highly successfully organized screening programme in the Republic of Korea provides a unique opportunity to conduct population-based studies within the framework of the programme. This will not only help maintain and further improve the quality of the programme, but also provide valuable evidence for other countries considering the potential of gastric cancer screening to reduce the burden of disease in their populations.