INTRODUCTION
Endoscopic ultrasound-guided fine-needle aspiration cytology (EUS-FNA) has been used in clinical practice for nearly 2 decades.1 EUS-FNA enables detailed imaging and sampling of both intramural and extramural structures and abnormal lesions of the gastrointestinal tract and of various intra-abdominal, mediastinal, and pelvic organs. Computed tomography (CT)-guided adrenal gland sampling is sometimes associated with adverse events. A few studies, each involving a limited number of patients, have demonstrated that EUS-FNA biopsy of the left adrenal gland is feasible.2,3 EUS-FNA of the left adrenal gland is safe and accurate4,5 and has a very favorable profile compared with the percutaneous approach because the only organ traversed by the needle is the gastric wall. However, because of the retrocaval location of the right adrenal gland, EUS-FNA of the right adrenal gland is rather difficult. EUS identified the left adrenal gland in almost all cases (98%) and the right adrenal gland in only 30% of the cases.6 Eloubeidi et al.7 reported successful sampling of the right adrenal gland with EUS-FNA in four patients with suspected metastatic disease in the right adrenal gland. The adverse event rate of the conventional percutaneous approach can be significant. In a review of 83 percutaneous CT or ultrasound guided adrenal biopsy, Mody et al.8 reported adverse event rate of upto 8.4%. Welch et al.9 evaluated the adverse event rate of 1,000 CT-guided biopsies and reported that the adrenal gland was the most common site of adverse events. Thus, EUS-FNA plays a definite role in establishing diagnosis of adrenal masses. In the current study, we reported our experience of EUS-FNA of the adrenal glands.
MATERIALS AND METHODS
The aim of this study was to examine the role of EUS-FNA in the evaluation of adrenal masses in cases where other imaging modalities failed or were not feasible. In this study, all patients had failed aspiration or no safe access with other imaging techniques (ultrasound or CT). This was a single-center prospective observational study of 21 patients who underwent EUS-FNA of adrenal masses between October 2010 and September 2013. The endosonographic and FNA features of these patients were observed.
Procedure
All patients provided written informed consent for EUS-FNA. This study was approved by the Institutional Review Board of Medanta Institute of Digestive and Hepatobiliary Sciences, Medanta. The instruments used were echoendoscopes (FG 34 UX, Pentax, Tokyo, Japan; and GF-UCT140 AL5, Olympus, Tokyo, Japan) with a longitudinal convex ultrasound transducer with an adjustable ultrasonic frequency of 5, 7.5, or 10 MHz, in combination with an ultrasound scanner (EUB 6500; Hitachi, Tokyo, Japan). The procedure was performed under conscious sedation using midazolam (3 to 5 mg intravenously) and pentazocine (10 to 30 mg intravenously). The left adrenal gland was identified using a linear echoendoscopeby tracing the descending aorta to the celiac trunk (usually located about 45 cm from the incisor teeth) and thenrotating the echoendoscope clockwise; the left adrenal gland appeared as a "seagull"-shaped structure. Right adrenal gland imaging was commenced in the duodenum with the echoendoscope in the long position along the greater curvature of the stomach. The inferior vena cava or the right kidney was then visualized, and the right adrenal gland was identified between the upper pole of the right kidney and the inferior vena cava. Sampling right adrenal was more difficult, from second part of duodenum, shortened scope position. After the optimal puncture site was determined, a puncture was made using a 22-gauge needle (SonoTip II; Medi-Globe, Achenmuhle, Germany), guided by real-time EUS imaging as described previously by us.9 EUS-FNA was performed at least three times per session (median, four times; range, 3 to 7). The aspirated material was expelled onto glass slides by reinsertion of the stylet and by using air flushes afterward. The needle was vigorously flushed with 10 mL of normal saline solution before each puncture, and heparin flushing was also performed if blood clots were found in the previous puncture. The aspirated material was macroscopically assessed, and whitish parts of the material were collected and placed in a formal in solution for pathologic examination. Some aspirated samples were smeared onto glass slides for cytologic examination. Ziehl-Neelsen staining for acid-fast bacilli (AFB) was performed. There was no pathologist orcytologist presentatthe site.
Diagnosis of tuberculosis (TB) was based on the presence of (1) caseating granulomas, (2) AFB, and (3) positive culture for Mycobacterium tuberculosis in the EUS-FNA specimens.10
Definite TB
(1) Granulomatous inflammation and (2) positive AFB staining/mycobacterial culture. The presence of a positive tuberculin test was considered as supportive evidence of TB and was not used as the sole criterion.
Presumptive TB
(1) Granulomatous inflammation, (2) other clinical signs suggestive of TB (fever, weight loss, night sweat, fatigue, and elevated erythrocyte sedimentation rate), and (3) negative AFB staining/mycobacterial culture; or (1) presence of granulomatous inflammation alone with (2) a positive Mantoux skin test and clinical suspicion of TB.
Statistical analysis
Data were analyzed using SPSS version 21 (IBM Co., Armonk, NY, USA). When data were normally distributed, continuous variables were compared using the Student t-test. Quantitative data are presented as mean and standard deviation with 95% confidence intervals. Categorical data are presented as proportions. The chi-square test was used to examine any statistical association between categorical variables.
RESULTS
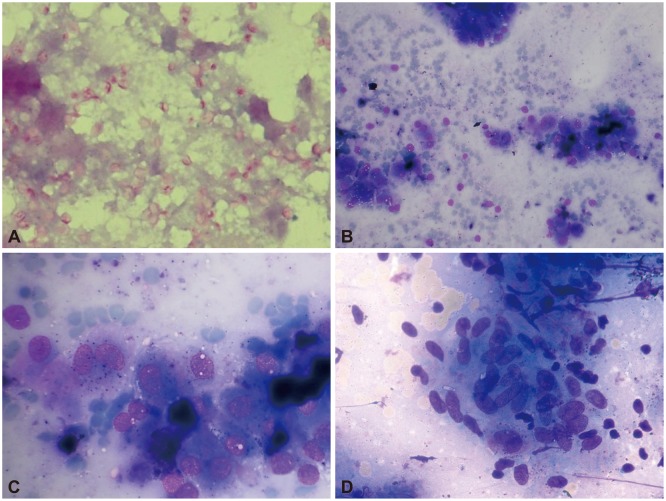
The study group included 21 patients (mean age, 56┬▒12.2 years; male:female ratio, 2:1), of which 12 (61.9%) had pyrexia of unknown origin and the rest underwent evaluation for metastasis. Three patients had unilateral right adrenal enlargement (one had lungcarcinoma, one had histoplasmosis, and one had adrenal lipoma), one patient had unilateral left adrenal enlargement (adrenal myelolipoma), and the remaining patients had bilateral adrenal enlargement. EUS-FNA was performed from the left adrenal gland in 18 patients (85.7%) and the right adrenal gland in three patients (14.3%). Adequate material was aspirated in all of them. Most of the 12 patients (61.9%) with fever of unknown etiology had constitutional symptoms (weight loss [n=8], loss of appetite [n=3], and fatigue [n=7]). Ten patients (47.6%) had caseating granulomas in the aspirate, and four of these patients were positive for AFB (Table 1). Thus, four patients had definite TB and six patients had presumptive TB. All six patients with presumptive TB had a positive Mantoux test, and in five of these patients, there was evidence of lymphadenopathy at other sites. Four patients had small mediastinal lymph nodes and one had mesenteric lymph nodes (these were small and inaccessible). Of the remaining patients, two were diagnosed with histoplasmosis (Gomori methenamine silver staining revealed abundant small intracellular budding yeast cells, morphologically consistent with Histoplasma spp., and adrenal myelolipoma in one patient) (Fig. 1). Fungal cultures from both adrenal EUS-FNA samples that showed evidence of Histoplasma spp. grew Histoplasma capsulatum. Nine patients underwent adrenal FNA to rule out metastasis from lung malignancy or hepatocellular carcinoma (as part of disease staging); EUS-FNA was positive for metastatic malignancy in seven of these patients (lung carcinoma [n=6], hepatocellular carcinoma [n=1]), and one patient with lung carcinoma had adrenal lipoma and one patient had adrenal myelolipoma. Positron emission tomography-CT (PET-CT) was performed in all patients diagnosed with malignancy, and the adrenal gland was 18F-fluorodeoxyglucose-avid in all these patients.
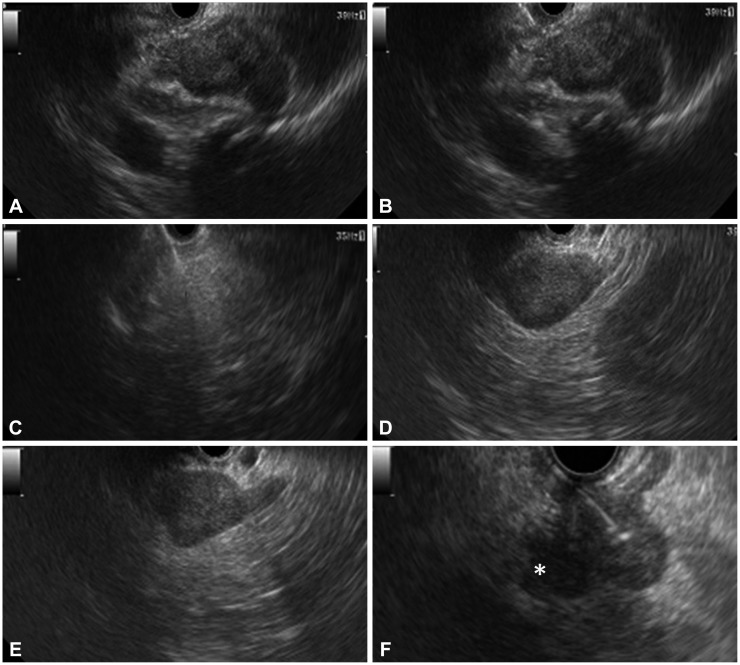
The median size of the adrenal gland was 2.4├Ś1.6 cm (range, 1├Ś1 to 3├Ś3.5). EUS showed a hypoechoic mass in most patients, except in those with TB (heteroechoic) and lipoma (hyperechoic) (Fig. 2). In patients with TB and adrenal histoplasmosis, the contour of the gland was maintained. In patients with other etiologies, there was an alteration in the shape of the adrenal gland with focal lesions and enlargement of only one limb. No significant intralesional calcification was observed in any of these patients. No procedure related-adverse events were encountered in any of these patients. All TB patients received the standard 4-drug antituberculosis therapy (World Health Organization category 1; isoniazid, rifampicin, pyrazinamide and ethambuol for 2 months followed by isoniazid and rifampicin for 4 months) and remained asymptomatic during follow-up. Patients with histoplasmosis were treated with amphotericin (intravenously) for 1 week, followed by treatment with oral itraconazole, 200 mg by mouth twice daily, for 1 year. We performed an adrenal function assay in one patient with histoplasmosis (the assay was performed only in this patient who alone clinically presented with adrenal insufficiency). Pyrexia of unknown origin improved with amphotericin therapy in this patient; however, adrenal insufficiency was persistent at 6 months of follow-up. Patients diagnosed with lung carcinoma and hepatocellular carcinoma received treatment as decided by the multidisciplinary panel, and the diagnosis remained unchanged till the end of follow-up. Patients with adrenal metastasis had disseminated malignancy precluding any curative resection.
DISCUSSION
Our study demonstrates the successful application of EUS-FNA in the evaluation of adrenal masses in cases where conventional imaging methods failed to yield a diagnosis. Using this procedure, satisfactory samples could be obtained and a diagnosis could be made successfully in all patients. EUS has been reported to be successful in imaging the left adrenal gland in almost all cases (98%) and the right adrenal gland occasionally (30%).6 Several EUS-FNA studies of various organs have successfully shown that traversing the wall of the stomach or duodenum with a 22-gauge needle has no serious consequences. In contrast, adverse events of the conventional percutaneous approach include hemorrhage, pneumothorax, pancreatitis, adrenal abscesses, bacteremia, and needle tract metastases.8 Welch et al.9 evaluated the adverse event rate of 1,000 CT-guided biopsies and reported that the adrenal gland was the most common site of adverse events (4/11 adverse events). In one study evaluating 277 consecutive adrenal biopsies over a 10-year period, 15 major adverse events occurred in eight of the 277 cases, and of these eight biopsies, five were of the left adrenal gland, and three were of the right adrenal gland. The adverse events were hematomas, one requiring adrenalectomy. No deaths were recorded in the study.11
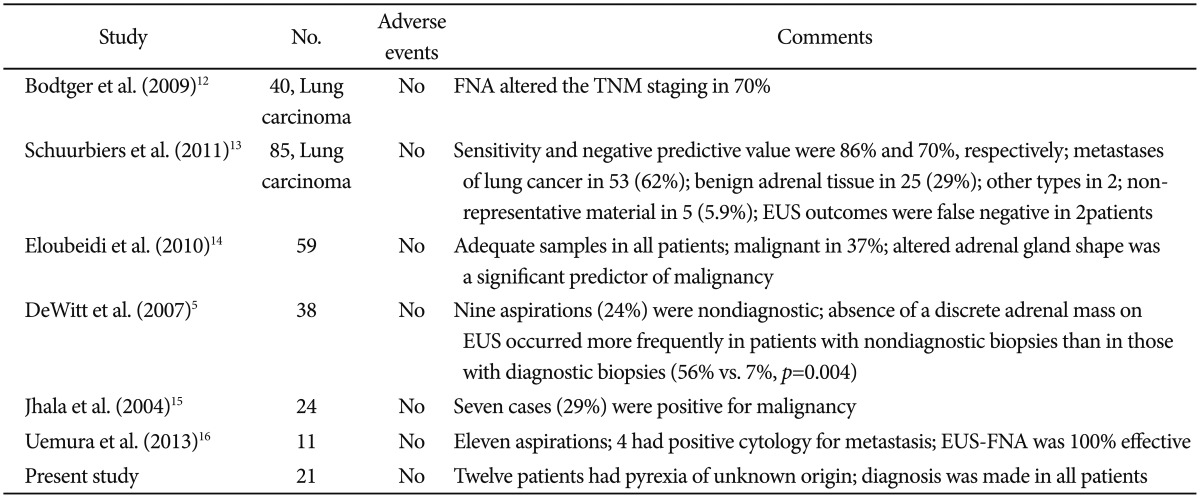
Our study showed results consistent with those of other studies reporting that EUS-FNA is a very safe procedure (Table 2).5,12,13,14 Bodtger et al.12 showed that EUS-FNA altered the TNM staging in patients with lung carcinoma. Eloubeidi et al.14 described a series of 59 patients who underwent EUS-FNA of the adrenal gland. The median adrenal gland size was 25├Ś17 mm. Adrenal tissue was adequate for interpretation in all patients. EUS-FNA confirmed malignancy in 22 patients (37%). Patients with malignant cytology had higher standard uptake values on PET than those with benign adrenal masses (p<0.001). Patients with malignant masses were more likely to have an altered adrenal gland shape compared to those with benign masses (crude odds ratio [OR], 12.0; p<0.001). Multivariable analysis showed that an altered adrenal gland shape was the only significant predictor of malignancy (adjusted OR, 7.94; p=0.015), whereas a size of 30 mm or larger (adjusted OR, 1.30; p=0.77) and hypoechoic nature of masses (adjusted OR, 12.05; p=0.15) were not significant predictors.14 Jhala et al.15 reported that EUS-FNA was a part of evaluation for metastasis in 24 patients, and seven patients (29%) were reported to be positive for malignancy. All patients diagnosed with metastatic carcinoma were confirmed on subsequent follow-up. Similar to that in our study, EUS-FNA of the right adrenal gland in one patient revealed myelolipoma. In 16 patients, benign adrenal cells were noted in EUS-FNA biopsy specimens from enlarged adrenal glands, and in five patients (31%), signs of adenoma were noted. In their study, morphology alone was not sufficient to distinguish between adrenal adenoma and adrenal hyperplasia.15 In another retrospective analysis of 150 patients, EUS-FNA was performed in 11 patients. 16 Findings suggestive of metastasis in either one or both of the adrenal glands were observed in six patients (4.0%) by CT, in five patients (3.3%) by PET-CT, and in 11 patients (7.3%) by EUS. EUS-FNA was performed simultaneously in the 11 patients, and in four patients, a diagnosis of metastasis was established. The accuracy of the diagnosis of adrenal metastasis was 100% with EUS-FNA, 96.0% with CT, and 97.0% with PET-CT (p=0.11). Our study represents the largest series of adrenal TB diagnosed by EUS-FNA. EUS features of the adrenal masses were observed to overlap with features of other etiologies, and EUS appearance alone was not sufficient to make a particular diagnosis. However, the sample size was limited.
In conclusion, EUS-FNA of adrenal lesions is safe and successful in patients in whom the conventional imaging methods have failed to yield a diagnosis. Although the right adrenal gland is conventionally believed to be a difficult site for EUS-FNA, it can be successfully sampled using this method. Tubercular involvement of the adrenal glands is a rare entity. EUS-FNA is a safe and effective method for diagnosing adrenal TB, and it helps in the prompt institution of antituberculous treatment. The EUS appearance of the adrenal glands as a sole criterion was not suggestive of any particular etiology, and EUS-FNA yielded conclusive diagnosis.