INTRODUCTION
The gastrointestinal (GI) tract is a rare site for metastatic lung cancer. The majority of esophageal metastases are caused by direct invasion of tumors originating from adjacent organs.1 Gastric metastases are even rarer, which implies that simultaneous metastasis to the stomach and esophagus from lung cancer is an extremely rare event. Review of previous case studies, most of which were autopsy cases, shows that most patients with GI metastases experienced no relevant symptoms.2
Here, we report a case of esophageal and gastric metastases from lung cancer presenting with symptoms of dysphagia and melena.
CASE REPORT
A 68-year-old man was diagnosed with stage IV lung cancer (adenocarcinoma) with the primary lesion in the left lower lobe and metastases to lymph nodes, bones, and the adrenal glands. He had experienced symptoms of left chest wall pain and dyspnea for a month. He had undergone an upper GI endoscopy as a regular health-screening test a few months before the diagnosis of cancer, and the initial esophagogastroduodenoscopy (EGD) showed no specific abnormalities.
The patient was referred to Seoul National University Hospital for further work-up and treatment, and he underwent chemotherapy for metastatic lung cancer, along with radiotherapy for spinal metastatic lesions.
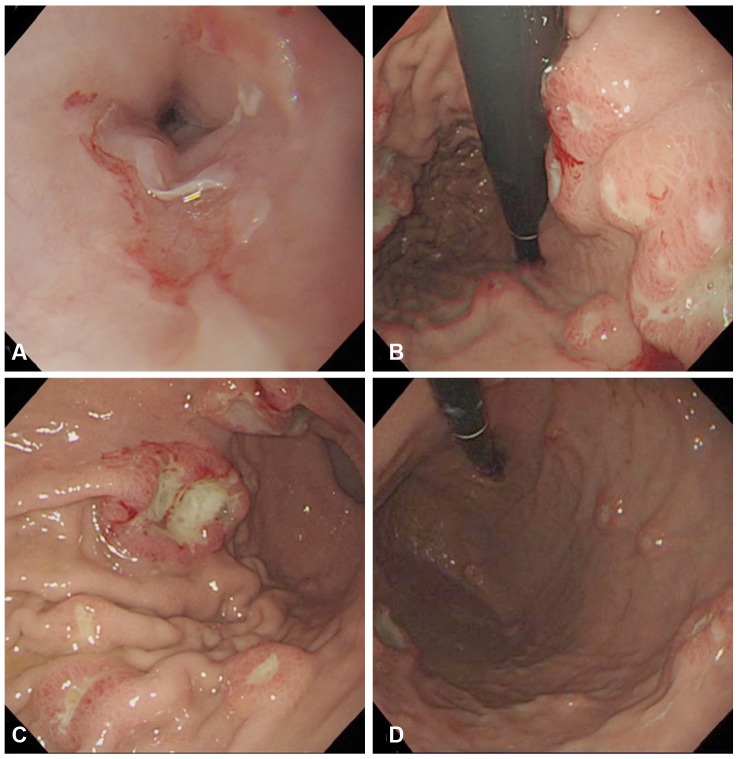
During a regular visit to the outpatient clinic, the patient complained of newly developed melena and dysphagia. To evaluate the GI tract, EGD was performed, and this revealed multiple lesions in the esophagus and stomach. Geographic erosion with epithelial break and mild hemorrhagic change without evidence of active bleeding was discovered at the distal esophagus, 35 cm from the upper incisors. Multiple volcano-shaped sessile masses with umbilication at the central portion were scattered through the entire stomach, from the fundus to the proximal antrum, measuring 0.5 (the smallest) to 5 cm (the largest) (Fig. 1).
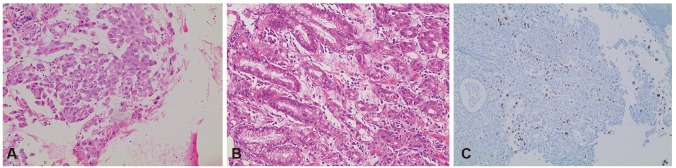
Endoscopic biopsies were performed at each lesion location, and the histopathological results showed poorly differentiated adenocarcinomas in both. The histologic patterns of the esophageal and gastric metastatic lesions were similar to those of the right lower paratracheal lymph node (4R) lesions, but to determine more precisely whether the lesions were primary esophageal and gastric cancer masses or metastases from the lung cancer, immunohistochemical (IHC) staining was additionally performed. The IHC results showed positive staining for thyroid transcriptional factor-1 (TTF-1) (Fig. 2). These results support the conclusion that the esophageal and gastric lesions were metastases that originated from the lung cancer.
Despite continued chemotherapy, the disease continued to progress. The patient eventually died 5 months after the initial diagnosis.
DISCUSSION
Lung cancer is the leading cause of cancer deaths in both men and women worldwide. Approximately 57% of patients present with metastatic disease at the time of diagnosis, with reported a 5-year survival rate of 4%.3 The most frequent sites for distant metastasis of lung cancer are the liver, adrenal glands, bones, and brain.4 In contrast, metastasis of lung cancer to the GI tract is a relatively rare event in the clinical setting. Rates of metastasis to the esophagus and the stomach are low, with reported incidences of 6.4% to 7.8% and 0.2% to 1.7%, respectively, based on clinical and autopsy findings.256 Meanwhile, a review of autopsy cases revealed that GI metastases from primary lung cancer are not so uncommon. According to the literature, the rate of GI metastasis from lung cancer ranges up to 11.9% to 14%, but this unexpectedly high incidence might be augmented by cases of local extension of the primary tumor to the GI tract.27 In Korea, there have been a few reports, to date, of GI metastasis from other solid organ tumors, such as hepatocellular carcinoma, breast cancer, lung cancer, and melanoma. There have also been some case reports of concurrent metastasis in the stomach and the small bowel, in the small and the large bowels, and in the stomach and the bowels. As far as we know, however, this is the first case report of simultaneous metastases to the esophagus and stomach from lung cancer diagnosed on endoscopy, in Korea.
Most patients with metastases to the upper GI tract have no relevant symptoms. Even when symptoms are present, they are usually nonspecific. Anemia and upper GI bleeding were the most common clinical presentations in patients with metastases to the upper GI tract.89 The patient in the present case showed both anemia and melena, and these findings led to the detection of the upper GI tract metastatic lesions. Therefore, clinicians should pay attention to GI signs among cancer patients because they are sometimes related to advanced metastatic cancer.
A volcano-like lesion or a mass with umbilication on the top (the "bull's-eye" or "target lesion" sign) is known as a classic characteristic of the radiographic findings of metastatic gastric cancer, as was first suggested by Pomerantz and Margolin10 in 1962. Since then, more reviews of the morphological characteristics of gastric metastatic lesions have been performed. It is generally agreed that metastatic involvement of the stomach can be divided into three distinct patterns on the basis of the endoscopic findings. These include solitary polypoid submucosal mass, multiple polypoid submucosal masses that may ulcerate, and infiltrating constricting patterns such as linitis plastica.8101112 In an analysis of 401 cases of metastatic gastric cancer, the gastric metastatic lesions in most cases were located in the middle or upper third of the stomach, and particularly on the greater curvature. In addition, solitary metastases were more common than multiple lesions in gastric metastasis.13
Pathologically, it is difficult to distinguish between a metastatic lesion to the stomach from a primary lung cancer and original gastric cancer, in the case of adenocarcinoma. However, using IHC staining, we can detect the primary site more easily and accurately.8 TTF-1 is a known IHC marker for lung and thyroid carcinomas. The sensitivity of TTF-1 in lung adenocarcinomas was 57.5% to 76%, and the specificity was 99% to 100% for primary lung carcinomas, in a previously reported series.14 In our case, because of the marker's high specificity, the positive result of the TTF-1 IHC staining provides strong evidence for the lung origin of the esophageal and gastric lesions, in addition to the clinical and morphological findings.
Generally, the presence of esophageal or gastric metastasis is related to advanced disease.5 In such clinical settings, the prognosis is poor and the expected survival period is relatively short. According to previous reports, the average time to death from the diagnosis of GI metastasis in lung cancer patients was 130.3 days, demonstrating a poor prognosis.15 However, constant improvement in the prognosis of cancer patients owing to advances in chemotherapy, as well as improvements in diagnostic tools, has resulted in the increased diagnosis of metastatic disease. While rare, GI metastasis from lung cancer can cause severe complications such as massive GI bleeding, perforation, and obstruction. In such cases, a longer survival or a more favorable outcome could be achieved with palliative resection of the metastatic site.161718
In summary, concurrent metastasis at the esophagus and stomach from lung cancer is very rare. Because most patients with metastases to the upper GI tract lack specific symptoms, clinicians should pay attention to the symptoms and signs of the patients, which can be important clues to the presence of GI metastases. IHC staining can be of help in determining whether a GI tumor is a primary cancer or a metastatic lesion.