Efficacy and Safety of Endoscopic Papillary Balloon Dilation Using Cap-Fitted Forward-Viewing Endoscope in Patients Who Underwent Billroth II Gastrectomy
Article information
Abstract
Background/Aims
Endoscopic exploration of the common bile duct (CBD) is difficult and dangerous in patients with Billroth II gastrectomy (B-II). Endoscopic papillary balloon dilation (EPBD) via a cap-fitted forward-viewing endoscope has been reported to be an effective and safe procedure. We analyzed the technical success and complications of EPBD in patients who underwent B-II.
Methods
Thirty-six consecutive patients with B-II were enrolled from among 2,378 patients who had undergone endoscopic retrograde cholangiopancreatography in a single institute in the last 4 years. The EPBD procedure was carried out using a cap-fitted forward-viewing endoscope with 8-mm balloon catheters for 60 seconds. We analyzed the rates of CBD exploration, technical success, and complications.
Results
Afferent loop intubation was performed in all patients and selective cannulation of the bile duct was performed in 32 patients (88.9%). Complications such as transient hypoxia were observed in two patients (5.6%) and perforation, in three patients (9.7%). The perforation sites were ductal injury in two patients and one patient showed retroperitoneal air alone without symptoms. Three patients manifested different clinical courses of severe acute pancreatitis and peritonitis, transient abdominal pain, and retroperitoneal air alone. The condition of one patient improved with surgery and that of the other two patients, with conservative management.
Conclusions
Patients with perforation during EPBD in B-II showed different clinical courses. Tailored treatment strategies are necessary for improving the clinical outcomes.
INTRODUCTION
Endoscopic retrograde cholangiopancreatography (ERCP) in patients who have undergone Billroth II (B-II) gastrectomy patients is a difficult and risky procedure because of altered anatomy and peritoneal adhesion. The success rate of ERCP is reported to be 50% to 88% in these patients, lower than the >90% success rate in patients with normal anatomy.1 The incidence of overall complications in these patients is 6% to 8%, higher than the rate of 1% to 5% in the general population.2
Many studies have reported how to overcome the problems associated with B-II gastrectomy. Forward-viewing endoscopy is useful for afferent loop intubation but selective cannulation is difficult.3 A recent study showed that cap-fitted forward-viewing endoscopy can help overcome this pitfall and that it was an effective and safe procedure.45 After selective cannulation, the papillary sphincter needs to be cut or stretched for orifice widening. Endoscopic sphincterotomy (ES) and endoscopic papillary balloon dilation (EPBD) have different advantages and disadvantages. ES is superior to EPBD for stone removal and prevention of acute pancreatitis, whereas EPBD is useful for patients with bleeding risks.67 In B-II gastrectomy, the depth and direction of ES are difficult to handle owing to altered anatomy; the major papilla is located upside down and a standard ES catheter is forwarded to the posterior wall of the duodenum, while the papilla is viewed in the oblique view in forward-viewing endoscopy.8 Recent studies reported that EPBD was superior to ES for reducing bleeding in cases with altered anatomy, but the rates of pancreatitis were still higher with EPBD than with ES.9
The aim of this study was to analyze the effects and complications of EPBD via a cap-fitted forward-viewing endoscope in B-II gastrectomy.
MATERIALS AND METHODS
Patients
Thirty-six patients (male:female, 27:9; mean age, 74 years) who underwent B-II gastrectomy and EPBD via a cap-fitted forward-viewing endoscope, were enrolled in this study. They accounted for 1.5% of all the 2378 ERCP procedures carried out from 2010 to 2013 at the tertiary referral center of Chungbuk National University Hospital; their records were analyzed retrospectively. ERCP was performed for removal of bile duct stones in 28 patients, diagnosis and treatment of common bile duct (CBD) stricture in six patients, biliary cancer in one patient, and postoperative bile leakage in one patient. The following comorbidities were noted: diabetes mellitus in 12 patients, hypertension in 10 patients, coronary artery diseases or atrial fibrillation in six patients, chronic obstructive pulmonary disease or pneumonia in three patients, and cerebrovascular accident in one patient. Further, 15, 16, and five patients belonged to the American Society of Anesthesiologists class 1, 2, and 3, respectively. Before ERCP, most of the patients showed abnormal results on liver function tests with alanine aminotransferase at 128 IU/L, alkaline phosphatase at 639 IU/L, and bilirubin at 1.8 mg/dL (Table 1).
EPBD using cap-fitted forward-viewing endoscopy
ERCP was performed using cap-fitted forward-viewing endoscopy (GIF-Q260; Olympus Optical Co., Ltd., Tokyo, Japan). After bile duct cannulation with a standard catheter, the balloon catheters were positioned along with a 0.035-inch guide wire (Boston Scientific Corp., Natick, MA, USA) via the papilla. EPBD was performed using Hurricane RX (Microvasive Endoscopy Boston Scientific Corp., Natick, MA, USA; balloon length, 4 cm; maximum inflated outer diameter, 8 mm) and Fusion Titan (COOK Medical Inc., Winston-Salem, NC, USA; balloon length, 4 cm; maximum inflated outer diameter, 8 mm) instruments. Sphincter injury was minimized by slowly inflating the balloon to 2 atmospheres, gradually increasing the pressure until the waist disappeared, and then holding the maximum pressure for 60 seconds (Fig. 1). Additional ES was performed in the cases where the stones were larger than 15 mm using a rotatable sphincterotome (Autotome Rx cannulating sphincterotome, 4.4 F; Boston Scientific) or Iso-Tome (MTW Endoskopie, Wesel, Germany).

(A) Endoscopic view of a papillary balloon dilation through the transparent cap. The ampulla is seen in a reversed position in the cap-fitted forward endoscopic view in patients who had undergone Billroth II gastrectomy. (B) Endoscopic papillary balloon dilatation. The balloon is located over a guide-wire and inflated and dilation took 60 seconds.
After EPBD or ES, other procedures were performed depending on the patients' condition: stone removal using a dormia basket (Flower Basket V, Center Valley, PA, USA) and a balloon-tipped catheter (Escort II double lumen extraction balloon; Cook Endoscopy, Winston-Salem, NC, USA), brush cytology, and metal stent insertion. A bile-duct stent (1 pigtail polyethylene, 7 Fr, 5 cm) was placed if complete stone removal was not achieved in one session or if active cholangitis was observed. A pancreatic stent (1 pigtail polyethylene, 5 Fr, 5 cm) was inserted if there were ≥2 cannulations into the pancreatic duct for prevention of post-ERCP pancreatitis. All procedures were performed by two endoscopists who had performed ERCP for more than 10 years.
Measurements of clinical and endoscopic data
Bile duct diameter and stone size were measured at maximal points adjusted for radiographic magnification. The number of sessions of ERCP and total procedure time were measured. Technical success and complications related to ERCP were obtained by review of medical records and radiologic images. The rates of afferent loop intubation and selective cannulation were used as measures of technical success. Outcomes were evaluated with hospital days and mortality. Data regarding complications such as bleeding, perforation, infection, acute pancreatitis, and cardiopulmonary events were also obtained. The following aspects of patients with complications were analyzed: diagnosis, procedure time, complication detection time, and methods, management, hospital course, and the cause of the events.
RESULTS
Endoscopic procedures
Afferent loop intubation was performed in all patients and selective bile duct cannulation was performed in 32 patients (88.9%). EPBD was performed in all patients and additional ES was performed in four patients. One patient with CBD stone and three patients with benign biliary strictures with failed cannulation underwent successful rescue percutaneous transhepatic biliary drainage. In addition to EPBD, other procedures were performed: stone removal in 27 patients, brush cytology in four patients, insertion metal biliary stent in one patient, and insertion of temporary plastic biliary or pancreatic stent in 22 patients and four patients, respectively. The total duration of the procedure was 36±15 minutes and the mean diameter of the CBD stones was 13.9 mm (Table 2).
Stone removal
Half of the patients with CBD stones had a single stone, and 10 patients had stones larger than 1 cm. Immediate stone clearance was achieved in 22 of the 27 patients (81.5%). Six patients needed more than one ERCP session for complete stone removal. Four patients, who showed severe cholangitis, were treated with immediate biliary drainage and follow-up ERCP for stone removal. Two patients were treated with immediate biliary drainage and partial stone removal at the second ERCP session and complete stone removal at the third ERCP session (Table 2).
Complications and outcomes
Thirty-one patients showed no serious complications, except for transient hyperamylasemia in seven patients (22.6%). Cardiovascular events with transient hypoxia occurred in two patients. No major bleeding or infection occurred. Perforation was observed in three patients (9.7%) in the retroperitoneal space during EPBD for stone removal in two patients and brush cytology in one patient. Among them, two patients experienced acute pancreatitis. Two patients showed indications of ductal injury (type III) and one patient showed retroperitoneal air alone without other symptoms (Table 3). They showed improvement with surgery (one patient) and conservative management (two patients). Three patients showed different clinical courses from asymptomatic to severe peritonitis (Fig. 2). Patients without complications showed improvement after admission for 9.3 days.

Clinical Characteristics, Treatment, and Outcome Data of Patients with Perforation during Endoscopic Balloon Dilation
Perforation patient 1
This patient had stones in the narrow and angulated distal CBD. After a 1-hour-long procedure, stone clearance was achieved. After ERCP, severe abdominal pain developed but his plain abdomen image appeared normal. Laboratory tests revealed acute pancreatitis and conservative management was initiated. His symptoms became aggravated and abdominal computed tomography (CT) revealed massive fluid and air collection in the retroperitoneal space (Fig. 3A-C). He developed severe acute pancreatitis, peritonitis, and sepsis, and catheters were inserted in the bile duct, gallbladder, and peritoneal space via the percutaneous approach. However, his symptoms continued to be aggravated and he underwent cholecystectomy and T-tube exploration. After surgery, his condition improved gradually and he was discharged on the 85th hospital day.
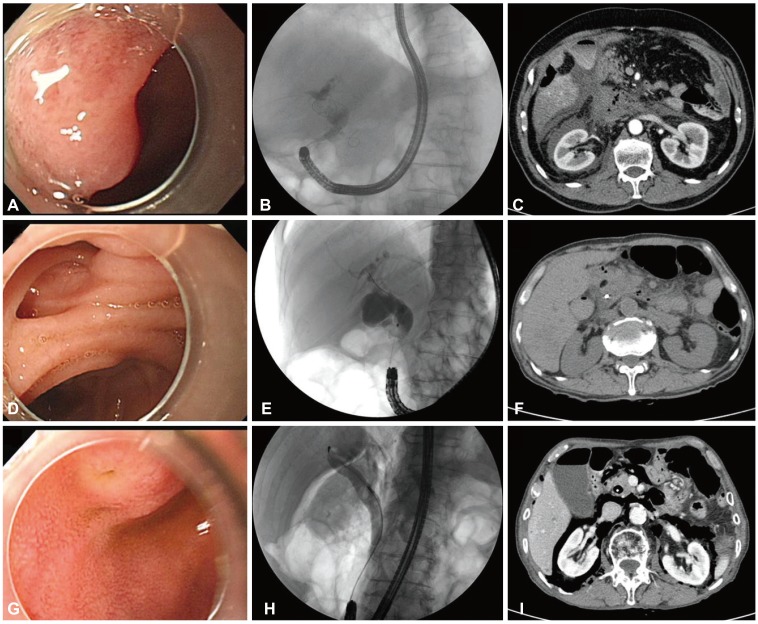
Endoscopic retrograde cholangiopancreatography findings of endoscopic view and cholangiography and computed tomography (CT) findings after perforations in three patients with Billroth II gastrectomy (A-C, patient 1; D-F, patient 2; G-I, patient 3). (A) A slit-like papillary orifice was seen in a reversed major papilla. (B) A single common bile duct (CBD) stone was seen in an angulated and narrowed distal CBD. (C) An abdominal CT showed massive fluid and air collection at the retroperitoneal space. (D) Periampullary diverticulum. (E) Distal CBD stenosis. (F) An abdominal CT revealed minimal air leak at the retroperitoneal space. (G) A papillary orifice with reversed major papilla. (H) A fluoroscopy showed a single round CBD stone and retroperitoneal air. (I) An abdominal CT showed massive air leakage without fluid accumulation in the retroperitoneal space.
Perforation patient 2
ERCP was performed to evaluate distal CBD stenosis and revealed a periampullary diverticulum. A 45-minute procedure was conducted involving brush cytology and insertion of a biliary stent. After ERCP, severe abdominal pain developed and abdominal CT revealed minimal air leak in the retroperitoneal space (Fig. 3D-F). Laboratory data revealed acute pancreatitis. His vital signs were normal and he improved after conservative management for 7 days.
Perforation patient 3
He underwent ERCP for acute cholangitis. During the procedure, retroperitoneal air leakage was detected. A biliary stent was inserted and the procedure was stopped. An abdominal CT showed massive air leakage without fluid accumulation in the retroperitoneal space (Fig. 3G-I). He had no symptoms and his vital signs were normal. He was discharged after close observation for 5 days.
DISCUSSION
This study showed that the use of a cap-fitted endoscope for EPBD in patients who had undergone B-II gastrectomy is an effective method for A-loop intubation and selective cannulation. EPBD with a conventional balloon catheter was also effective in these patients. However, perforation was observed during EPBD in some of the patients who had undergone B-II gastrectomy. The patients showed diverse clinical courses, ranging from an asymptomatic state to severe peritonitis. The management methods also differed according to the clinical manifestation, ranging from close observation and conservative treatment to surgery. The different clinical courses could be attributed to the differences in the time of diagnosis of perforation and injury site.
ERCP-related perforation is classified from type I to IV according to the perforation site as perforation involving the bowel, sphincter of Oddi, bile duct, or retroperitoneal air alone, respectively.10 Another classification method is based on perforation sites and causes, such as guide wire injury (group I), periampullary perforation (group II), and duodenal perforation (group III).11 Bowel perforation caused by endoscopy is typically large and traditionally requires operative management after prompt diagnosis, whereas ductal or paravaterian perforation caused by sphincterotomy or guide wire injury can be managed with non-surgical methods.1112 Recent studies reported that bowel perforation can also be managed with non-surgical methods such as an endoclip alone, combination of glue and endoloop, and band ligation with an additional clip.13
The perforation sites could not be identified directly in the present study, but the outer side of the duodenal wall of the sphincter of Oddi could have been the injury site. Compared to the inner side of the duodenal segment, which was surrounded by dense and thick smooth muscles, the outer duodenal segment was surrounded by rough and thin smooth muscles (Fig. 4). During EPBD, the distal bile duct was stretched, causing tiny tears.1415 Compressed air can leak in via these tiny tears in a limited space of the A-loop. We assumed that the injury was caused by the guide-wire or basket in patient 1 and by the balloon itself in patients 2 and 3, who were very thin. We assume that the smooth muscle of the outer duodenal wall of the sphincter of Oddi was too thin in these patients and thus susceptible to tearing caused by minimal balloon inflation.

(A, B) Normal histology of the sphincter of Oddi at the oral protrusion inside the muscle layer of the duodenum. The bile duct was surrounded by the dense and thick sphincter of Oddi (A, H&E stain, ×40; B, H&E stain, ×100). (C) Normal histology of the bile duct outside the duodenal wall (H&E stain, ×100). The bile duct is surrounded by the rough and thin sphincter of Oddi, which has partly disappeared on the side of the parenchyma of the pancreas. The blue and translucent circle, 8 mm in diameter, denotes maximal inflation of the balloon.
Early detection of perforation is necessary for reducing the likelihood of complications related to it. A previous study reported that late detection of retroperitoneal perforation required surgical intervention with a serious clinical course.16 Perforation could not be detected early in patient 1, as the patient's plain abdomen image showed no evidence of perforation. Therefore, the patient was diagnosed with post-ERCP pancreatitis. After 36 hours, an abdominal CT showed retroperitoneal fluid collection with perforation. Despite aggressive non-surgical management, his peritonitis continued to worsen. His condition improved after surgical intervention and laborious management. Patient 2 had only abdominal pain without evidence of an inflammatory response. Early detection of perforation was possible by abdominal CT, but not detected on a plain abdomen image. We believe that his abdominal pain was not caused by perforation but by acute pancreatitis. Patient 3 showed massive air leakage in the retroperitoneal space during the procedure. However, abdominal CT showed no evidence of fluid leakage and no symptoms were apparent. Retroperitoneal perforation related to EPBD is usually too small to be detected on a plain abdomen image; therefore, we recommend checking the abdominal CT without contrast enhancement when even minimal clues of perforation are present.
Methods to reduce the occurrence of EPBD-related perforation in B-II gastrectomy patients are yet to be identified. Consistent with previous reports, bowel perforation was not observed in this study. Although perforation rates were somewhat higher than those reported in other studies, serious perforation developed in only one patient, who had undergone a long procedure and showed no biliary drainage but the perforation was detected late. The others showed very stable clinical courses; they underwent short procedures, biliary drainage, and early detection was possible in their cases. No safe methods have yet been established. Some experts have recommended gradual inflation, lower maximal pressure, and short balloon time. However, others reported that long balloon times can help reduce pancreatitis. Further studies are needed to establish optimal EPBD methods.
Many studies have reported that biliary drainage can be useful for reducing bile leakage in cases of minor retroperitoneal perforation.17 However, insertion of a biliary endoprosthesis in all patients is not practical in clinical settings. We recommend performing biliary drainage in cases of patients at risk of perforation, namely patients with a history of ES or B-II gastrectomy, intramural injection of contrast material, prolonged procedure time, dilation of biliary stricture, and sphincter of Oddi dysfunction.1819 In addition to the above risk factors, further studies should be conducted to determine whether patients with low body mass are at a high risk of developing perforation.
This study showed that the fatal course of perforation is not due to the amount of leaked air but due to the amount of fluid collected. Consistent with the present study, a few studies have reported about asymptomatic air leakage. After ES, an abdominal CT revealed asymptomatic retroperitoneal air in six out of 21 patients (29%). One study suggested the likelihood of leakage of compressed air via co-existing mucosal disruption or small iatrogenic perforation.20 One case report indicated that retroperitoneal air occurred with subcutaneous emphysema following ES without symptoms.21 These air leaks are not a cause for alarm and require no surgical intervention.
In conclusion, perforation was not a rare occurrence during EPBD-related procedures in patients that had undergone B-II gastrectomy. Preventive biliary drainage and early detection of perforation can prevent the development of a fatal course in these patients. When perforation is suspected, physicians need to make an early diagnosis and decide upon surgery or conservative management. Treatment must be tailored according to the sites and severity of EPBD related perforation.
Acknowledgments
This work was supported by the research grant of Chungbuk National University in 2013.
Notes
Conflicts of Interest: The authors have no financial conflicts of interest.