INTRODUCTION
Acute gastric dilatation is a rare condition with several possible etiologies, including eating disorders (e.g., anorexia nervosa and bulimia), postoperative complications following abdominal surgery, electrolyte disturbances, and mechanical obstruction due to a foreign body, tumor, or pyloric stenosis [1-8]. Although complications of acute gastric dilatation, such as gastric necrosis or perforation, shock, sepsis, and abdominal compartment syndrome, are very rare, death may result if complications do occur. Therefore, early diagnosis and prompt treatment are very important in the management of acute gastric dilatation, particularly since delayed treatment can result in respiratory failure, shock, sepsis, gastric necrosis, or perforation. In rare cases, acute gastric dilatation is caused by decreased gastric motility resulting from bacterial toxins in patients with diabetes mellitus or ParkinsonŌĆÖs disease who suffer from respiratory infections such as pulmonary tuberculosis or pneumonia [8].
Here, we report a case of delayed pneumoperitoneum and acute pulmonary edema caused by acute gastric dilatation in a patient with ParkinsonŌĆÖs disease.
CASE REPORT
A 78-year-old woman with ParkinsonŌĆÖs disease, cerebral lacunar infarction, and hyperlipidemia presented to the emergency department of our hospital with a chief complaint of fever and general weakness. The Eastern Cooperative Oncology Group performance status for this patient was grade 3.
She had developed a cough with fever 2 days before admission, followed by anorexia, altered mental status, and general weakness. She visited the local medical clinic for treatment, at which time her blood pressure was 70/40 mm Hg, and her body temperature was 38.5┬░C. She was immediately transferred to our Emergency Department for advanced care. In the emergency room, her blood pressure was 90/48 mm Hg, pulse rate was 88 beats per minute, respiratory rate was 20 breaths per minute, body temperature was 38.0┬░C, and oxygen saturation was 91% in room air. Her mental status was drowsy. She complained of mild abdominal pain and dyspnea. Physical examination revealed crackles over the left lower lung field. The abdomen was massively distended with reduced bowel sounds. Diffuse mild tenderness was noted in the entire abdomen.
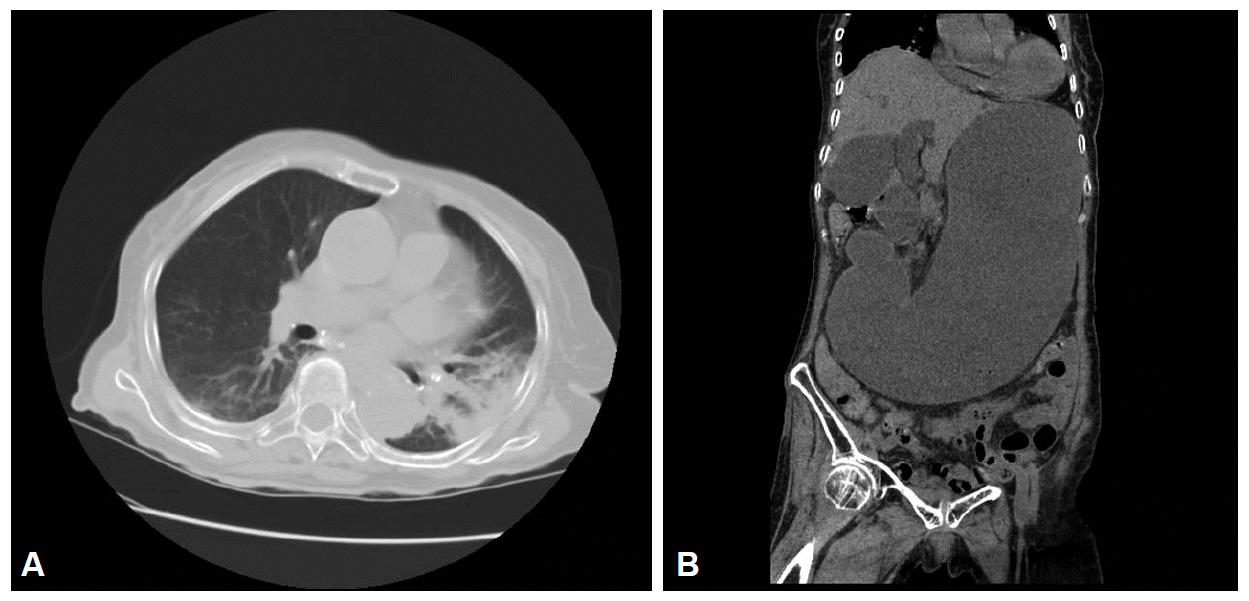
The initial laboratory evaluation provided the following results: white blood cell count, 8,850 cells/mm3 (neutrophils, 90.3%); hemoglobin, 10.2 g/dL; hematocrit, 30.6%; platelet count, 182├Ś103 cells/mm3; erythrocyte sedimentation rate, 42 mm/hr; C-reactive protein, 19.41 mg/dL; blood urea nitrogen, 78.9 mg/dL; procalcitonin, 3.75 mg/mL; and creatinine, 2.46 mg/dL. Arterial blood gas analysis showed a pH of 7.502, pCO2 of 22 mm Hg, pO2 of 63.5 mm Hg, HCO3 of 17.4 mmol/L, and SaO2 of 94.5% with oxygen at a rate of 2 L/min via a nasal cannula. A thorax computed tomography (CT) scan without contrast revealed pneumonic infiltration in the lower lobe of the left lung (Fig. 1A) and severe distension of the stomach from the upper abdomen to the pelvis without the presence of free air (Fig. 1B). The blood culture did not show any growth.
She was diagnosed with acute gastric dilatation in the presence of pneumonia. Intravenous fluid replacement, intravenous infusion of empirical antibiotics (400 mg moxifloxacin and 2 g cefotaxime sodium) once every 24 hours, vasopressor administration, and oxygen supply via a nasal cannula were initiated. A nasogastric tube was inserted, and 4 L of a dark brown liquid was drained through the tube.
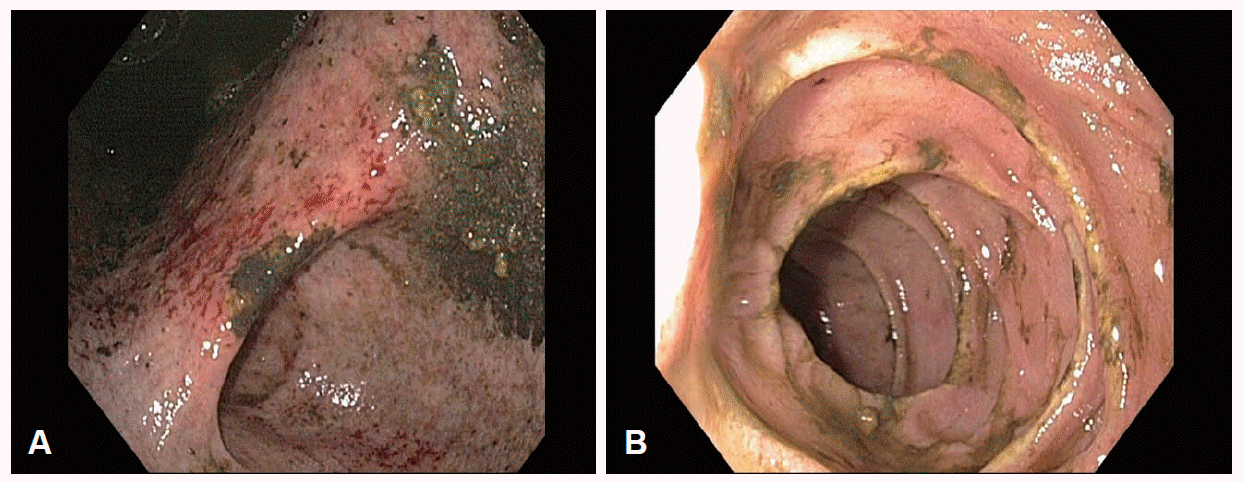
After the initial treatment, her vital signs became relatively stable and her mental status improved. The drainage volume of the liquid from the stomach gradually decreased. An esophagogastroduodenoscopy (EGD) was performed 24 hours after admission, and the remnant food and liquid material in the stomach (1,500 mL) was drained. EGD revealed acute gastric mucosal erosion, multiple gastric superficial ulcers, and the absence of gastric outlet obstruction, duodenal ulcers, or necrosis (Fig. 2).
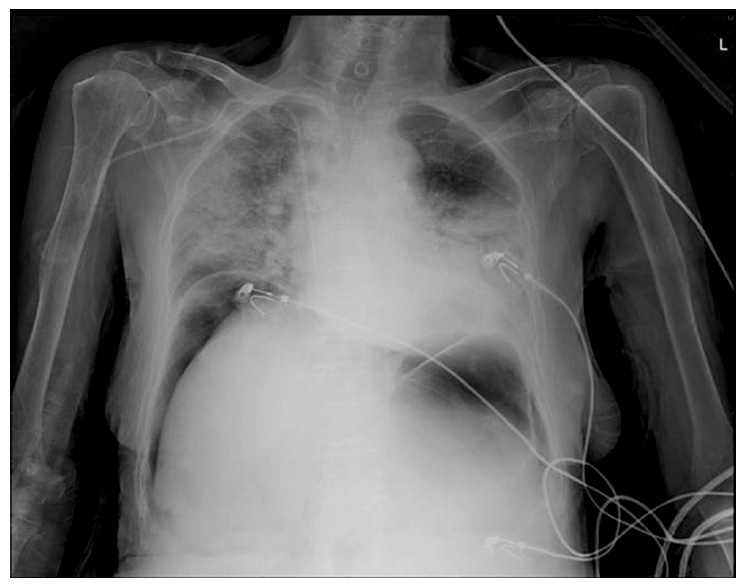
Approximately 36 hours after admission, the patient suddenly complained of severe dyspnea, chest pain, and aggravated abdominal pain. Her blood pressure was 90/50 mm Hg; pulse rate, 106 beats per minute; oxygen saturation, 86%; and breathing oxygen, 10 L/min through a non-rebreather mask. We immediately performed a follow-up plain chest radiography, which revealed free air in both subphrenic spaces and pulmonary edema in both lung fields (Fig. 3). Her general condition worsened rapidly with a dramatic decrease in her mental condition and blood pressure along with aggravated dyspnea, which resulted in the death of the patient.
DISCUSSION
Acute gastric dilatation can be caused by conditions such as eating disorders, trauma, gastric hypomotility related to diabetic autonomic neuropathy, and mechanical obstruction [1-8]. However, the pathogenesis of this condition remains largely unknown and several theories have been suggested [4,6,8]. The atony theory states that the stomach in a patient with an eating disorder becomes atonic and atrophic during fasting, and sudden ingestion of a large amount of food in this state may result in acute gastric dilatation because of the aggravation of an already weakened stomach. According to the mechanical theory, acute gastric dilatation is caused by gastric outlet obstruction related to superior mesenteric artery syndrome, pyloric stenosis, presence of a foreign body, or gastric cancer, among other causes. Acute gastric dilatation may also be caused by regional alterations that occur with biliary tract disease, pancreatitis, or appendicitis [9]. It can also be the result of aerophagia, which is caused by the relaxation of the upper esophageal sphincter, usually during anesthesia or due to debilitation after surgery [2]. With gastric hypomotility, as is present with diabetes mellitus or ParkinsonŌĆÖs disease owing to gastric autonomic neuropathy [1-8], bacterial toxins produced in patients with respiratory infections (e.g., pneumonia or pulmonary tuberculosis) may lead to acute gastric dilatation [8].
Our patient had ParkinsonŌĆÖs disease and she presented with symptoms of pneumonia and was diagnosed with acute gastric dilatation on the abdominal CT. The patient showed no gastric necrosis or pyloric stenosis during the EGD, but later developed pneumoperitoneum and acute pulmonary edema resulting in rapid deterioration of her medical condition and eventual death. Although we did not observe a gastric perforation during laparotomy or autopsy, we suspect that the pneumoperitoneum resulted from the aggravation of previous multiple superficial gastric ulcers and erosions induced by ischemia. The other possibility is that gastric micro-perforation was masked by the large amount of remnant food and liquid material in the stomach. These perforations might have clearly been observed if air insufflation during the EGD was performed.
Our patient did not undergo any imaging study to evaluate complications after EGD because she did not develop any symptoms. However, if initial endoscopic evaluation is limited by food residue in the stomach or is performed in patients who are less expressive about changes of their symptoms, follow-up endoscopy or abdominal imaging studies should be performed to recognize complications of acute gastric dilatation, such as gastric ischemia or necrosis, even if patients do not have any signs or symptoms. We think that the acute pulmonary edema, which was considered to be noncardiogenic pulmonary edema, was caused by septic shock.
The first step in the development of gastric perforation is necrosis of the gastric mucosa; when the necrosis advances to all layers of the stomach, perforation can occur. The stomach receives sufficient blood supply, and hence, gastric ischemia and necrosis very rarely occur. However, ischemia of the stomach wall due to venous insufficiency may develop when the intragastric pressure exceeds 20 mm H2O [10-12]. Spontaneous perforation and necrosis of the stomach is more common in women (67%) and frequently occurs along the lesser curvature of the stomach (63%) [7,12].
Leukocytosis or azotemia on laboratory analysis may suggest complications of acute gastric dilatation, such as ischemia or necrosis. The common presenting symptoms of acute gastric dilatation are emesis and diffuse abdominal distension with mild tenderness; however, severe pain is uncommon [7,13]. It is occasionally accompanied by hypotension or tachycardia as signs of shock, which may be caused by decreased venous return owing to the compression of the inferior vena cava by increased intra-abdominal pressure.
Plain radiography of the abdomen may indicate a distended stomach or free air in the right subphrenic spaces without any evidence of a small bowel gas shadow. Abdominal CT is the most useful diagnostic tool for visualizing gastric distension and observing small amounts of free intraperitoneal gas and associated abdominal organic lesions [4,14]. EGD is used to evaluate pyloric stenosis and the general condition of the gastric mucosa (i.e., the presence of ischemia and necrosis) [7,14].
The initial treatment consists of prompt nasogastric decompression, fluid resuscitation, and correction of electrolyte imbalance. Intragastric pressure can be decreased with only partial decompression in most patients, which is usually followed by an improvement in the symptoms of acute gastric dilatation [2,12]. Prompt emergency exploration must be performed if nasogastric decompression fails or in the presence of suspected peritonitis or abdominal compartment syndrome. The surgical methods may include surgical decompression, duodenojejunostomy, or partial or total gastrectomy [2,5,6,15,16].
In most cases, severe sepsis is already present at the time of diagnosis in patients with gastric perforations. In these cases, surgical interventions result in poor outcomes, including a mortality rate as high as 33% to 65% in patients who receive aggressive surgical treatment, such as laparotomy. However, the mortality rate is nearly 100% in patients with complications, including gastric perforation or necrosis, who not receive a surgical intervention [7,12,17].
In conclusion, early recognition and nasogastric decompression are important for the treatment of acute gastric dilatation. Prompt surgical intervention should be considered when gastric perforation or necrosis is suspected, as it can significantly reduce the patient mortality rate. Immunocompromised patients with chronic illness require close observation even when they do not show any symptoms suggestive of complications. In particular, if initial endoscopic or abdominal radiologic findings show no gastric ischemia or necrosis, follow-up EGD or abdominal imaging studies are essential to recognize complications of acute gastric dilatation at an early stage.