Endocuff-Assisted Colonoscopy—A Novel Accessory in Improving Adenoma Detection Rate: A Review of the Literature
Article information
Abstract
Endocuff (Arc Medical Design) is a U.S. Food and Drug Administration-approved device that is attached like a cap to the distal tip of the colonoscope; it is used to improve adenoma detection rates during colonoscopy. The aim of this review was to summarize and evaluate the clinical and technical efficacy of Endocuff in improving adenoma detection rate. A comprehensive literature review was performed to identify studies describing this technique. In this review article, we have summarized case series and reports describing Endocuff use and results. The reported indications, results, limitations, and complications are discussed.
INTRODUCTION
The adenoma detection rate (ADR) is an important quality measure of screening colonoscopy delivery and is crucial for reducing colorectal cancer (CRC)-related morbidity and mortality. ADR is defined as the percentage of patients aged 50 years or older who are undergoing first-time colonoscopy for CRC screening, who have at least one adenoma detected and removed [1,2]. The ADR differs widely among populations, reaching approximately 22% in a large screening population [3]. Up to 25% of adenomas are reportedly missed during colonoscopy because of poor colon preparation and poor visualization behind mucosal folds. Flat lesions in the right side of the colon can be particularly difficult to detect. ADR is an important quality measure because it is inversely associated with the risk of interval CRC. Recent studies estimate that each 1% increase in the ADR lowers the risk of interval cancers by 3% [4-6].
Several endoscopic innovations and devices have been developed to increase the ADR, with either limited benefit (e.g., visual enhancement technologies, cap) or high technical impact (Third Eye Retroscope; Avantis Medical Systems, Sunnyvale, CA, USA; 330° full-spectrum endoscopy).
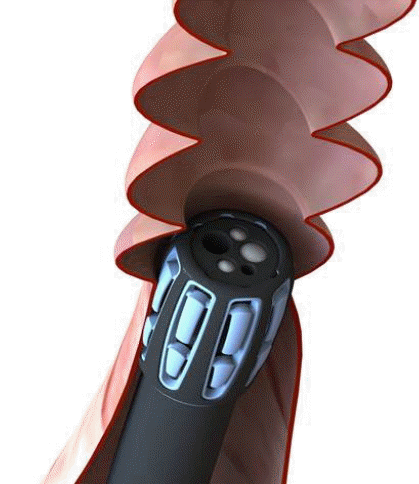
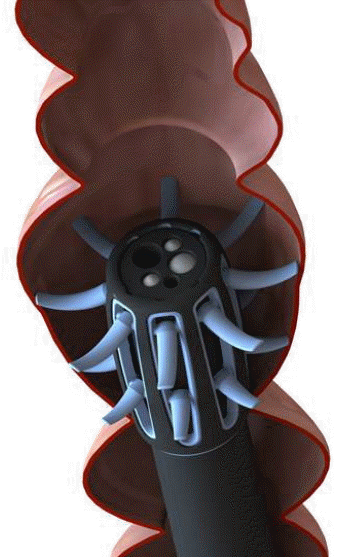
Since 2012, a new endoscopic device called Endocuff (EC; Arc Medical Design, Leeds, United Kingdom) has been available (Figs. 1, 2). EC is a U.S. Food and Drug Administration-approved device that is attached like a cap to the distal tip of the colonoscope. The device has eight flexible branches arranged in two rows. These branches are used to flatten the folds of the colon while withdrawing the colonoscope, allowing for improved visibility behind the folds [7].
There are limited recent publications summarizing the clinical and technical success of EC in improving ADR during colonoscopy. In the present review, we review the published literature and describe the indications, results, limitations, and complications reported with EC.
MATERIALS AND METHODS
Extensive English language literature search was conducted using PubMed, Medline, and Google to identify peer-reviewed original and review articles by using the keywords ‘Endocuff,’ ‘adenoma detection rate,’ ‘colonoscopy,’ and ‘colorectal cancer.’ Only human studies were included. The references of pertinent studies were manually searched to identify additional relevant studies. The indications, procedural details, technical and clinical success rates, complications, and limitations were considered as part of the inclusion criteria. Search results yielded mostly small sample sized prospective studies and case reports, which limited statistical analysis in the form of meta-analysis. None of the authors has any conflicts of interest or financial relationships with the company that produces or distributes the device described in this review article.
RESULTS
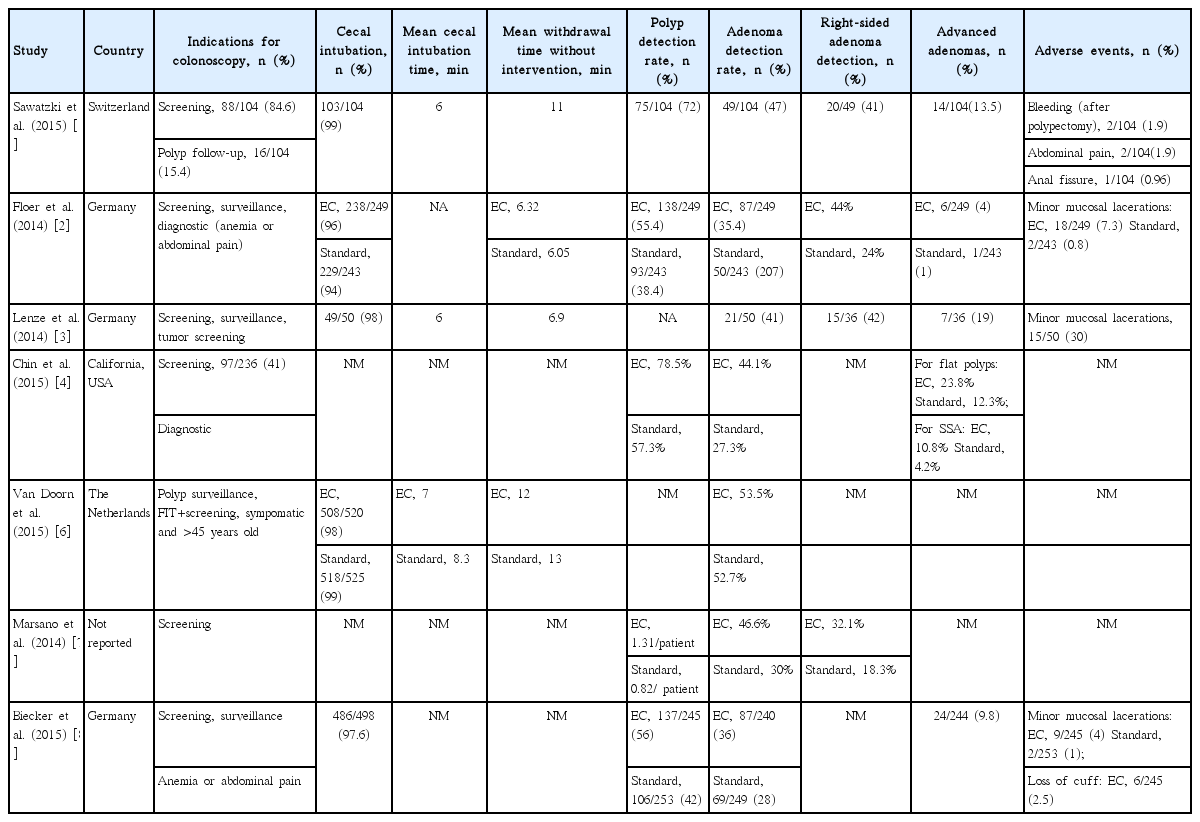
Seven original published articles were considered appropriate for inclusion. All seven articles were case series. Tables 1, 2 summarize the included studies.
Demographics
As shown in Table 1, most of the published cases were reported from European countries. A total of 2,761 patients were included across all studies. Five of the seven studies reported the gender of the patients included in the study. Those five studies reported that 49.2% (1,086/2,207) were female and 50.8% (1,121/2,207) were male. Mean age calculated from all reported cases was 62.4 years (Table 1).
Indications
Indications for undergoing colonoscopy included screening for CRC, surveillance of previously detected polyps, or diagnosis of symptoms such as abdominal pain or anemia [1-4,6-8]. Van Doorn et al. [6] also included patients with positive fecal immunochemical testing results in their screening cohort.
Technical and clinical findings/success rates
Five of the seven studies included were comparative studies [2,4,6-8]. Patients were randomized into either a standard colonoscopy group or an Endocuff-assisted colonoscopy (EAC) group. In four of these five studies, the EAC group had a significantly higher ADR (Table 2). In the study by Van Doorn et al. [6], the ADR between the standard and EAC groups was similar and EC did not seem to increase the accuracy of colonoscopy. For those studies that included procedural data, cecal intubation was reported in 97% of patients undergoing EAC. Withdrawal time and cecal intubation time did not significantly differ between the EAC and standard colonoscopy groups; however, the studies reporting this data were very few for a conclusion to be made. Polyp detection rate (PDR) was significantly higher in EAC groups as compared to standard groups across all comparative studies, regardless of indication for colonoscopy. ADR was also superior with EC in detection of right-sided adenomas in those studies that reported location of adenomas. Furthermore, for studies that reported polyp histology, advanced adenomas were more frequently detected in the EAC group than in the standard group. In the study by Sawatzki et al. [1], advanced adenomas were detected and removed in 13.5% patients. These included 11 adenomas greater than 10 mm, two tubulovillous adenomas, and one neuroendocrine carcinoid. In the study by Floer et al. [2], a statistically significant difference was shown in the advanced ADR for non-invasive low-grade dysplasia in the EAC group versus the standard group. The technical success rate was 100% across all studies using the EC assisted approach, and no major complications were noted. Most studies did not measure clinical success rates, as the patients undergoing the procedure were predominantly asymptomatic at the time of screening colonoscopy.
Complications and adverse outcomes
Four studies reported minor complications, as summarized in Table 2 [1-3,8]. The study by Biecker et al. [8] was the only study that reported loss of the cuff as an adverse outcome of the procedure. The majority of included studies noted minor mucosal bleeding and post-procedural bleeding as the most common complication.
Limitations
Thus far, clinically successful cases have been published with few complications reported, but this may be due to a publication bias, as the procedure is fairly new. As more technically and clinically relevant cases are published, additional data may become available for assessment regarding the impact of EAC on colon cancer-related morbidity and mortality.
DISCUSSION
Colonoscopy performed as a part of colon cancer prevention programs remains the gold standard for CRC screening. The ADR seems to be most crucial in preventing interval colon cancer, as a suboptimal ADR is significantly associated with a higher incidence of CRC. An ADR of at least 20% is generally accepted as sufficient for colon cancer prevention programs and should be the standard for colon cancer prevention centers [9-11]. Recently, many studies have aimed to achieve a better ADR through various accessory devices (cap assistance, third eye retroscope, EC) and advancements in endoscope design (full spectrum endoscopy system, balloon-assisted colonoscopy, confocal laser endomicroscopy). As a simple attachment to the distal tip of the colonoscope, the EC is an effective and inexpensive alternative for increasing the ADR without restricting the field of vision. The benefit of EC is that it is a safe measure that improves PDR and ADR in a screening population with no severe adverse events, even in patients with diverticulosis. Furthermore, the cecal intubation time is not lengthened, and a significant number of small polyps can be detected in the right side of the colon [12,13].
Other technical innovations, like cap-assisted colonoscopy (CAC), back-to-back colonoscopy, or narrow band imaging, have also been evaluated for higher polyp and ADRs. CAC shows conflicting results in terms of improving ADR. In a study by Rastogi et al. [10], ADR improved with CAC; in contrast, a recently published two-center randomized controlled trial conducted by de Wijkerslooth et al. [14] failed to show an improvement in ADR. When compared to EC, more training is likely to be necessary due to the technical nature of CAC and its effect on the view of the examiner [8]. Furthermore, during CAC, fecal material can become trapped in the cap and impair the visual field; repeated cleaning maneuvers can be time consuming [14]. In our review of pertinent articles, there was no mention regarding the learning curve timing for precise use of EAC. Most endoscopists included in these case series were experienced and had performed a large volume of colonoscopies prior to use of EAC.
Another upcoming device aimed to increase ADR is the NaviAid G-EYE System (SMART Medical Systems Ltd., Ra’anana, Israel), which includes the G-EYE balloon colonoscope and the NaviAid inflation system. The NaviAid G-EYE colonoscope permanently integrates a balloon onto the flexible tip of a standard colonoscope and is inflatable, reusable, and re-processable. The integrated balloon can be inflated by the endoscopist upon colonoscope withdrawal, to carry out balloon-assisted colonoscopy. The mechanical flattening and straightening of the colonic mucosal folds with the inflated balloon allows visualization of hidden anatomical areas; thus, increasing the ADR [15]. In a prospective study by Hasan et al. [16], the diagnostic yield of balloon-assisted colonoscopy versus that of standard forward-viewing colonoscopy in the detection of simulated polyps in a colon model was compared. Median PDR for all simulated polyps was significantly higher with the G-EYE balloon colonoscope as compared to that with standard forward-viewing colonoscopy. In the first human study using G-EYE, Gralnek [15] carried out a single-center, prospective cohort study that looked at patients referred for CRC screening, polyp surveillance, or diagnostic evaluation. A total of 44 polyps in 25 of 47 subjects were identified by the G-EYE balloon colonoscope, resulting in a 53.2% PDR. The investigators reported that 36/44 (81.8%) polyps were ‘adenomas,’ with 21/47 subjects having at least one adenoma, yielding a 44.7% ADR. Gralnek [15] concluded that G-EYE appeared to be safe and feasible to use, as the primary end point of this study was safety and efficacy of the device. In a multicenter, randomized, controlled trial comparing standard colonoscopy with G-EYE balloon colonoscopy, Shpak et al. [17] reported that when compared with standard colonoscopy, G-EYE balloon colonoscopy detected an additional 17 adenomas, representing 81% more than that in the group undergoing standard colonoscopy. Furthermore, there was only a 7.5% adenoma miss rate reported with balloon colonoscopy [16-18].
As shown in our review article, EAC might have some advantages over standard colonoscopy. Our findings suggest that EAC may result in higher overall ADR and PDR and may be more effective in detecting right-sided adenomas and allowing better inspection of proximal colonic folds. Detection of these polyps that are more likely to be visualized hidden, especially on the reverse side of the mucosal folds, promoted higher ADR and PDR in the EAC group in the studies included in our review. Findings also suggested that EC might facilitate easier polypectomy. This could be as a result of higher friction that allows for avoidance of sudden slippage of the colonoscope because the distal row of EC arms can be used to expose the polyp. Therefore, polypectomy procedures seem to be easier due to a more stable colonoscope position and better visualization by EC. Additionally, EC does not impair the direct wide-angle view of the colonoscope. Finally, in the studies included in our review, no major complications were reported for EC and its use in screening colonoscopy [5-7,8].
CONCLUSION
In conclusion, the EC is a new, safe, and promising attachable device for screening colonoscopy. As a result of our literature review, the EC seems to provide benefits over standard colonoscopy in facilitating complete colonoscopy, improving the visualization of the colonic mucosa, detecting hidden polyps, and improving the overall PDR and ADR. The use of EC is safe and feasible. Recently, the EC device has undergone additional development, with longer arms (EC vision) with the potential to improve ADR further [8].
As previously discussed, improving the ADR correlates with a decrease in colon cancer risk. Future follow-up studies are necessary to demonstrate the impact of EAC on colon cancer-related morbidity and mortality before broad use of the EC device in colon cancer prevention programs can be recommended.
Notes
Conflicts of Interest: The authors have no financial conflicts of interest.