INTRODUCTION
Pancreatic thoracic pseudocysts and pancreaticopleural fistulas are rare complications of acute and chronic pancreatitis. These conditions have symptoms that are often misleading. Moreover, no uniform diagnostic and treatment standards exist. We present a case of a patient with sepsis secondary to infected ascending mediastinal pancreatic pseudocysts, which are a rare complication of endoscopic retrograde cholangiopancreatography (ERCP). Thereafter, we present effective treatment methods and discuss optimal management strategies.
CASE REPORT
A 43-year-old man with bronchial asthma and three episodes of acute pancreatitis (AP, suspected etiology due to steroids) presented with exertional dyspnea approximately 1 month prior to admission. There was no evidence of AP on admission; the last episode had been 6 months before. The patient was in generally good health. Laboratory tests revealed mild anemia (hemoglobin 12.0 g/dL) and thrombocytosis (platelets 677У109/L).
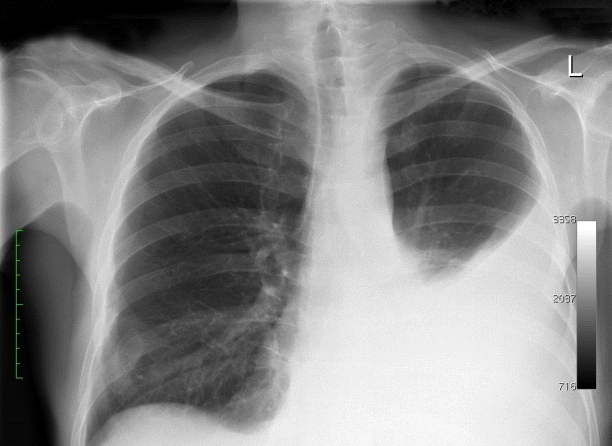
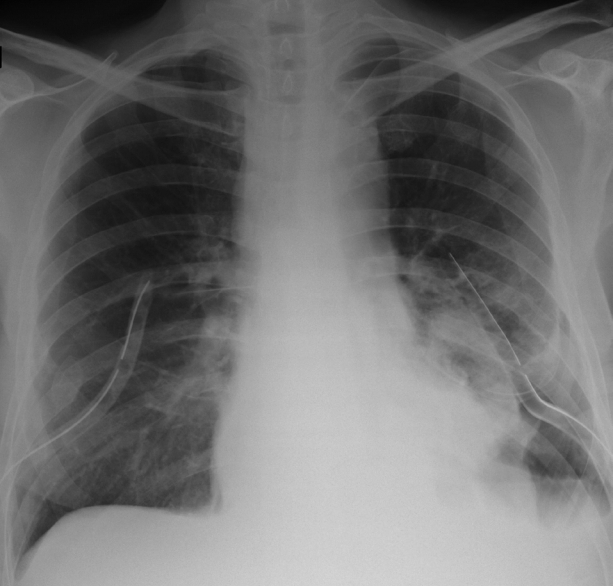
A chest radiograph showed fluid in the left pleura reaching the fifth rib in the mid-axillary line (Fig. 1). An abdominal computed tomography (CT) scan revealed pancreatic pseudocysts that communicated with the mediastinum. Thoracocentesis was performed. This resulted in 900 mL of a clear, yellowish fluid with a high amylase (14,449 IU/L) and protein (3.5 g/dL) content. The diagnosis of a pancreaticopleural fistula was made. A two-stage approach was chosen to manage the fistula: first, pancreatic ductal stenting of the leakage site via ERCP, followed by transgastric or transduodenal endoscopic pseudocyst drainage.
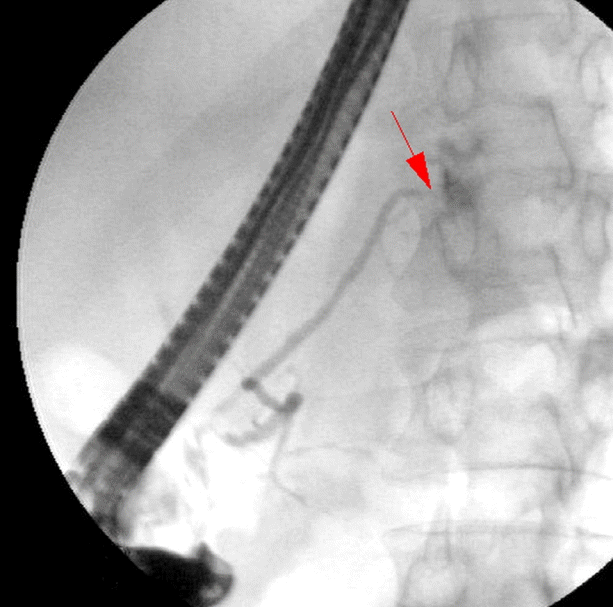
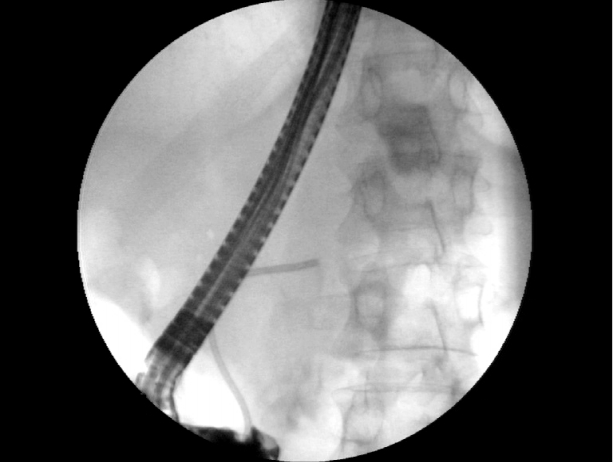
ERCP revealed leakage of contrast medium into the thoracic cavity in the proximal portion of the pancreatic tail (Fig. 2). A stent was placed in the pancreatic duct (Fig. 3). Initially, the patient started to recover after the procedure. However, on the second day after ERCP, he deteriorated rapidly and developed clinical signs of septic shock. Laboratory tests revealed elevated inflammatory markers, including a procalcitonin level of 10 ng/mL, and elevated serum (760 IU/L) and urinary amylase (4,416 IU/L).
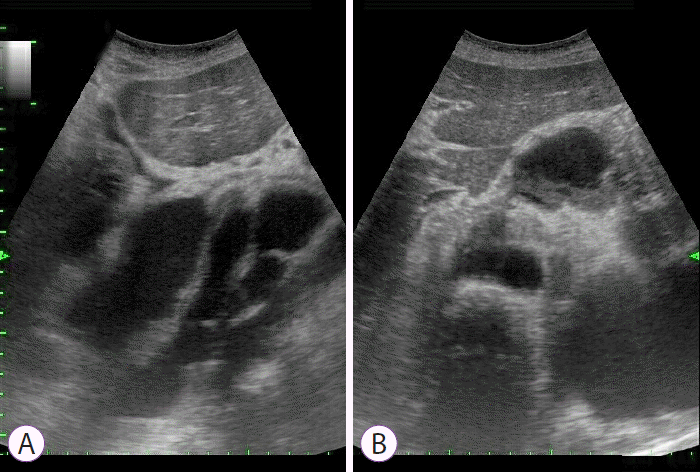
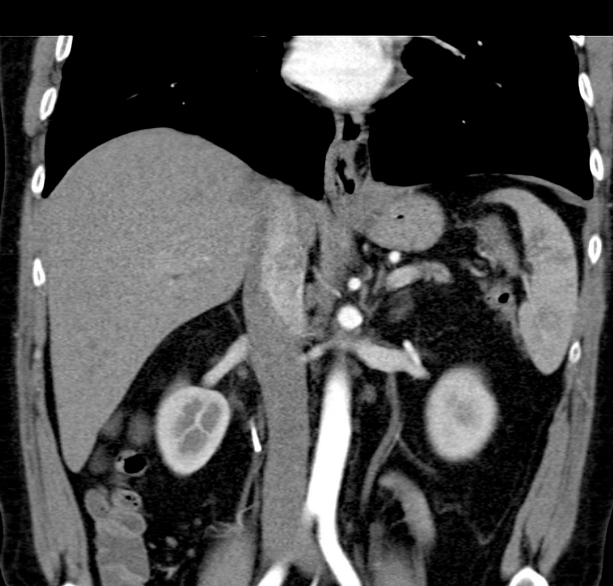
The patient improved clinically after fluid resuscitation and the administration of broad-spectrum antibiotics (meropenem). However, he continued to require intravenous amiodarone for persistent tachycardia. Continuous intravenous somatostatin and total parenteral nutrition were also administered. He developed dyspnea, chest pain, and vomiting, but further evaluation did not reveal any signs of myocardial infarction. An abdominal ultrasound (Fig. 4) and chest CT scan revealed posterior mediastinal pseudocysts extending from the pancreas through the diaphragmatic hiatus to the mediastinum and neck, with compression of the surrounding tissues and fluid in both pleural cavities (Fig. 5). Bilateral drainage of the pleural cavities was performed in the thoracic surgery ward. This comprised 2 weeks of active and 1 week of ambulatory passive drainage. Thereafter, chest radiography revealed regression of the pleural fluid (Fig. 6). The patientтs inflammatory markers improved, and he was discharged home after 3 weeks of staying in hospital in a satisfactory physical condition. A follow-up CT scan performed at 12 months showed that the pseudocysts and pleural fluid had resolved (Fig. 7). He remained symptom-free throughout the 26-month follow-up period.
DISCUSSION
Thoracic pancreatic pseudocysts and pancreaticopleural fistulas are rare complications of acute and chronic pancreatitis, as well as trauma [1]. They are triggered by the disruption of the pancreatic duct, which leads to pancreatic fluid penetrating through the diaphragmatic orifices (usually the aortic or esophageal hiatus) into the mediastinum. There, it can transform into a pleural fistula or a mediastinal pseudocyst [1,2]. To the best of our knowledge, there have been fewer than 100 cases of mediastinal pancreatic cyst expansion reported in the literature [3]. While the precise incidence is unknown, the frequency of pancreaticopleural fistulas in patients with pancreatitis is estimated to be 0.4% to 4.5% [4,5]. Approximately 80% of cases of pancreaticopleural fistulas are associated with mediastinal pseudocysts [6].
The symptoms are often misleading, and typically result from significant pleural effusions or the compression of mediastinal structures. Pulmonary symptoms such as dyspnea, cough, chest pain, or dysphagia, can be present, as well as life-threatening complications like hemoptysis, cardiogenic shock, and acute respiratory failure [7]. Abdominal symptoms such as pain are rare [5].
The diagnosis is typically established by multiple radiologic tests, including ultrasound, CT, magnetic resonance cholangiopancreatography (MRCP), ERCP, and pleural fluid analysis. The pleural fluid is typically characterized by a high amylase and albumin content [5]. The mean amylase level in the pleural fluid of patients with pancreaticopleural fistulas may exceed 10,000 IU/L [8]. ERCP and MRCP are helpful in establishing the fistulous communication between the pancreatic duct and pleura. ERCP leads to the diagnosis in 80% of cases, demonstrates the fistulous tract in 59%, and also allows for pancreatic duct stenting [9].
There are many strategies to treat thoracic pancreatic pseudocysts. These depend on the anatomy, pseudocyst size, and presence and severity of symptoms [7]. Conservative therapy with somatostatin analogs, total parenteral nutrition, and/or bromhexine hydrochloride [5,10,11] can be successful for asymptomatic patients with small cysts [10]. Large or symptomatic pseudocysts require invasive therapies such as surgery (distal pancreatectomy, pancreatic head resection, cystojejunostomy, cystogastrostomy, or Puestow procedure) or drainage [12,13]. Drainage procedures can be performed percutaneously under CT or ultrasound guidance [7,14]; alternatively, they may be performed endoscopically via the gastrointestinal tract wall through the transesophaegal, transgastric, or transpapillary approach [15,16]. The advantage of endoscopic treatment is the lower risk of complications compared to percutaneous drainage [7]. However, due to the small number of pancreatic pseudocysts described in the literature, there has not been any studies comparing the efficacy and safety of different types of endoscopic drainage [7].
Ajmera and Judge [7] presented an algorithm for the treatment of mediastinal pancreatic pseudocysts. They proposed that all unstable patients with life-threatening complications should be treated with open surgery, while the management of stable patients should depend on the size of the pseudocyst and the presence of symptoms. Symptomatic patients or those with large cysts should be treated with drainage. Endoscopic drainage is preferred, especially if the procedure is performed in a tertiary referral center and the anatomy is favorable, which means the cyst is located near the gastric wall or is communicating with the pancreatic duct. Asymptomatic patients should be treated conservatively and followed up [7]. Pseudocysts complicated by infection, obstruction, rupture, or hemorrhage should be treated surgically [7].
There have also been attempts to introduce thoracosurgical treatment methods for thoracic pancreatic pseudocysts. For example, Chang and Chen [17] has reported the successful use of video-assisted thoracoscopic surgery with bilateral drainage of the pleural cavity in treating ascending mediastinitis arising from pancreatic pseudocysts.
In cases of pancreaticopleural fistulas, suppression of exocrine pancreatic function with octreotide and ERCP with fistula stenting is optimal, and has a low morbidity and mortality rate [18]. Surgical treatment is more effective with a success rate of 90%; however, it is associated with a higher mortality rate, and therefore remains a second-line option [18]. Wronski et al. [4] proposed an algorithm to treat pancreaticopleural fistulas: all patients should be assessed with MRCP prior to treatment, and the treatment method should depend on the pancreatic ductal abnormality. Patients with non- or a mildly dilated pancreatic duct without stenosis should have a trial of conservative treatment for 2 to 3 weeks. If ductal strictures and ductal disruption in the pancreatic head or body are present, ERCP should be used as first-line treatment. If complete ductal obstruction or strictures and fistulas in the pancreatic tail are present, surgery should be considered the first-line treatment instead [4].
In this present case, we aimed to improve the patientтs clinical status and resolve both the pleural fluid and the pseudocysts. As previously described, a two-stage therapeutic strategy was employed: first, the location of the leak was stented by ERCP; second, the abdominal portion of the pseudocyst was drained through the gastrointestinal tract via the transgastric or transesophageal approach. However, in retrospect, the patient could have been more optimally assessed with MRCP and/or chest CT before ERCP took place. The patientтs post-ERCP course was complicated by life-threatening sepsis, which was initially misdiagnosed as AP secondary to the ERCP. However, the patient had dominating тchestт symptoms, including dyspnea, chest pain, and recurrent paroxysmal tachycardia. In addition, the ultrasound and CT scan showed polycyclic pseudocysts extending from the pancreas through the mediastinum to the neck beyond the scanning border with compression of surrounding tissues. This led us to the diagnosis of ascending post-ERCP mediastinal pseudocyst inflammation. Due to the patientтs unstable condition and the risk of life-threatening complications, he was transferred to the thoracic surgery ward, where effective bilateral pleural drainage was performed. Our treatment approach was based on the findings of the pleural fluid communicating with the mediastinal pseudocysts and the pancreatic duct. We also believed that a pancreatic duct stent would prevent further pseudocyst expansion.
The patientтs clinical status eventually improved, his symptoms resolved, and he was discharged home on day 21 after admission. His hospital stay consisted of 1 week in the department of gastroenterology, and 2 weeks in the department of thoracic surgery. A 12-month follow-up abdominal CT scan showed that the pseudocysts had resolved. To the best of our knowledge, this case of complete pseudocyst resolution after bilateral pleural drainage, preceded by stenting of the pancreatic duct, has not been described in the literature.
In summary, pancreatic duct stenting followed by bilateral pleural fluid drainage can be an effective treatment of thoracic pancreatic pseudocysts in the context of a coexisting pancreaticopleural fistula. We believe that every patient with thoracic pancreatic pseudocysts and/or pancreaticopleural fistulas should be carefully assessed by a multidisciplinary teamтincluding gastroenterologists, surgeons, thoracic surgeons, and radiologistsтto determine the optimal management strategy.