Management of Benign and Malignant Pancreatic Duct Strictures
Article information
Abstract
The diagnosis and management of pancreatic strictures, whether malignant or benign, remain challenging. The last 2 decades have seen dramatic progress in terms of both advanced imaging and endoscopic therapy. While plastic stents remain the cornerstone of the treatment of benign strictures, the advent of fully covered metal stents has initiated a new wave of interest in calibrating the pancreatic duct with fewer sessions. In malignant disease, palliation remains the priority and further data are necessary before offering systematic pancreatic stenting.
INTRODUCTION
Pancreatic duct stricture is a common problem that is associated with various etiologies. Benign etiologies include chronic pancreatitis, recurrent acute pancreatitis, trauma, surgical complications, and pseudocysts. Pancreatic strictures can also be a manifestation of malignancy [1,2]. The diagnosis and treatment of pancreatic strictures have proven challenging for physicians. Most pancreatic strictures present with persistent or recurrent abdominal pain, or with symptoms of chronic pancreatitis and exocrine insufficiency. The possibility of underlying malignancy necessitates investigation with high-quality cross-sectional imaging including computed tomography and magnetic resonance imaging/magnetic resonance cholangiopancreatography. Any masses that are appreciated on imaging should be followed by endoscopic ultrasonography with fine needle aspiration (EUS-FNA) [3].
EUS-FNA has replaced endoscopic retrograde cholangiopancreatography (ERCP) as the method of choice in obtaining tissue specimen, as it is associated with a higher success rate and fewer post-procedural complications [4]. Pancreatic brushing should be employed in the absence of a definitive mass on imaging [3]. Furthermore, repeat EUS has been shown to improve diagnostic yield in the case of continued suspicion of cancer and to increase the sensitivity of pancreatic mass detection in the setting of chronic pancreatitis [4,5]. Confocal endomicroscopy is another technology that aids in diagnosing neoplasms in indeterminate masses and in the mapping of abnormal pancreatic ducts prior to surgery [6]. Imaging and endoscopic diagnostic tests are ideally correlated with a careful evaluation of the patient’s history to establish the benign or malignant nature of the pancreatic strictures. Serum markers such as IgG4 in autoimmune pancreatitis or CA 19-9 in pancreatic cancer can also be useful in determining the etiology of the stricture.
TREATMENT
The treatment of a pancreatic stricture depends on whether it is benign or malignant. Asymptomatic pancreatic strictures can be left without intervention if malignancy has been excluded. Persistent symptoms such as abdominal pain or postprandial pain are the indications for intervention in benign pancreatic strictures. Factors that must be considered when selecting the modality of treatment include the patient’s individual characteristics such as age and comorbidities, the location and number of strictures, and finally, the expertise of the endoscopist in EUS and ERCP. Treatment of pancreatic strictures can be medical, endoscopic, or surgical. Medical treatment of pancreatic duct strictures includes abstinence from alcohol, strict adherence to a low-fat diet, small frequent meals, and pancreatic enzyme supplements to address symptoms of exocrine insufficiency [7].
Rapid advancements in endoscopic technology have put endoscopy at the forefront of the management of pancreatic strictures. The mainstay of endoscopic therapy for pancreatic strictures includes pancreatic sphincterectomy, followed by the dilation of pancreatic stricture and the placement of a pancreatic duct stent (Fig. 1). This sequence is associated with immediate pain relief in 65%–95% of patients and with sustained pain relief in 32%–68% of patients [8,9]. ERCP is also considered the first-line modality in the management of main pancreatic duct obstruction in the setting of chronic pancreatitis [10,11]. Dilation is usually done before stenting and can be performed using wire-guided balloons (4–6 mm), a bougie, or a Soehendra stent retriever [3]. Plastic stents have become the standard endoscopic treatment of main pancreatic duct strictures in the setting of chronic pancreatitis and they are usually left in place for a fixed duration of time or exchanged upon recurrence of symptoms [12,13]. In addition to plastic stents, self-expanding metal stents have also been successfully used in treating pancreatic strictures (Fig. 2); however, plastic stents are associated with more long-term follow-up data and experience [14]. The larger diameter of plastic stents also seems to confer an advantage in terms of the rate of hospitalizations for abdominal pain [15]. A high rate of stricture recurrence upon removal of the stent has been observed, with a 38% recurrence rate at the 2-year follow up and the need for repeat stenting [16]. The number of strictures and the location of the stricture seem to be critical determining factors in the success of endotherapy. A symptomatic chronic pancreatitis patient with main pancreatic duct stricture at the head of the pancreas would be the ideal candidate for ERCP with stenting. Strictures at the tail of pancreas are more difficult to treat endoscopically. Moreover, endoscopic treatment of multiple chronic pancreatitis-related strictures (chain of lakes appearance) is even more challenging [17].

The use of plastic stents in the treatment of benign pancreatic strictures. (A) Guidewire insertion. (B) Balloon dilation. (C) Plastic stent deployment.

The use of fully covered self-expanding metal stent (FCSMS) in treating benign pancreatic strictures. (A) Distal pancreatic stricture identified on fluoroscopy. (B) 8 mm × 60 FCSMS deployment. (C) Removal of the FCSMS at 3 months after deployment. Image showing stricture resolution on pressure injection.
The technical failure rates of ERCP range from 3%–10%. The most common reasons for technical failure include failure of cannulation of the main pancreatic duct, severe strictures, pancreatic stones, or altered anatomy [18-20]. In patients where conventional ERCP failed, EUS-guided drainage of the main pancreatic duct is emerging as a less invasive alternative to surgery. Our team reported a large, international multicenter study on the safety and efficacy of EUS-guided pancreatic drainage (EUS-PD) in patients who failed conventional ERCP therapy for pancreatic strictures. Among a total of 80 patients, technical success was reported in 89% and clinical success was reported in 81% [21]. Initial studies of EUS-PD have demonstrated a wide range of efficacy from 50%–100% [18-20,22,23], which is likely reflective of the inherent learning curve. Conventionally, surgery or percutaneous intervention would be the next step for patients who fail ERCP therapy for pancreatic strictures; however, those approaches are both associated with significant morbidities. Our multicenter, international investigation demonstrated a 20% adverse events rate, which is still significantly lower than the 30% adverse events rate reported with surgical interventions [21,24-26].
Pancreatic cancer is the fifth leading cause of cancer-related deaths in the western world [27]. Pancreatic strictures and main pancreatic ductal obstructions are common manifestations of pancreatic malignancy. The vast majority of pancreatic cancer patients are inoperable at the time of diagnosis and have a very poor prognosis [28]; therefore, the main concern is palliation. As in the case of patients with chronic pancreatitis, endoscopic stenting can be used in the decompression of malignant ductal obstructions that cause postprandial epigastric pain in patients with pancreatic cancer, particularly in patients with tumors in the head of the pancreas [29]. Both plastic and metal stents have been employed in the treatment of malignant pancreatic strictures [12]. Plastic stents have a reported patency of 2 months, which is attributed to their small diameter [30]. On the other hand, metal stents have demonstrated a longer duration of patency, fewer reinterventions, and increased cost-effectiveness in patients with a prognosis longer than 6 months [30,31]. Whether metal or plastic stents provide better results remains to be determined, and further data are also needed to ascertain the efficacy of fully covered self-expanding metal stents in relieving malignant ductal obstructions [30]. With both plastic and metal stents, technical success rates have ranged between 81% and 100%, and rates of pain improvement have ranged between 61% and 100% [12].
CONCLUSIONS
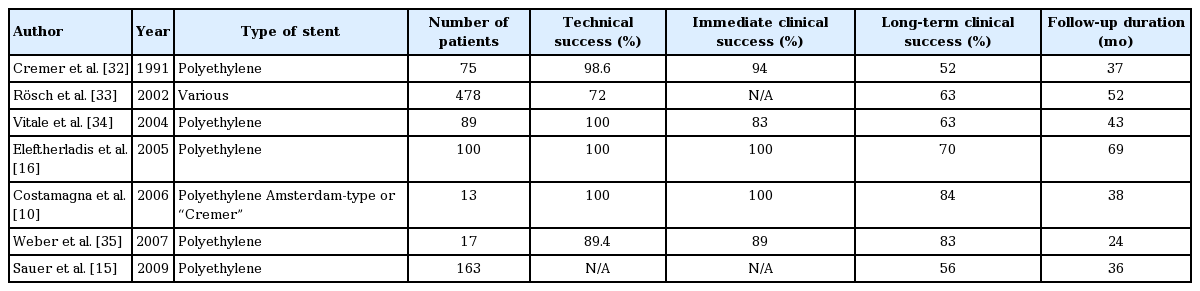
The diagnosis and treatment of pancreatic strictures can prove challenging, and a high index of suspicion should always be maintained in approaching patients with pancreatic strictures in order to rule out malignancy as the underlying etiology. High-quality imaging studies, patient history, and serum markers should be obtained in all patients, and the treatment modality of choice depends on the benign or malignant nature of the pancreatic stricture. Benign strictures can be left untreated if they are asymptomatic. Persistent abdominal pain and recurrent attacks of pancreatitis are among the indications for intervention. Patients in whom conventional ERCP endoscopic therapy for benign strictures fails should be offered EUS-PD as a safe, minimally invasive, and effective alternative to surgery, according to the availability of endoscopic expertise. Patients with malignant pancreatic strictures might benefit from palliation provided by the decompression of the pancreatic ductal obstruction, for which both plastic stents (Table 1) and metal stents (Table 2) have been used [10,15,16,32-43]. However, more data and longer follow up periods are needed to establish the superiority of one type of stent over the other. Patients with benign strictures should be followed up in 3- or 6-month intervals if malignancy is suspected.
Notes
Conflicts of Interest: The authors have no financial conflicts of interest.