De-novo Gastrointestinal Anastomosis with Lumen Apposing Metal Stent
Article information
Abstract
Gastric outlet obstruction, afferent or efferent limb obstruction, and biliary obstruction among patients with altered anatomy often require surgical intervention which is associated with significant morbidity and mortality. Endoscopic dilation for benign etiologies requires multiple sessions, whereas self-expandable metal stents used for malignant etiologies often fail due to tumor in-growth. Lumen apposing metal stents, placed endoscopically with the intent of creating a de-novo gastrointestinal anastomosis bypassing the site of obstruction, can potentially achieve similar efficacy, with a much lower complication rate. In our study cohort (n=79), the composite technical success rate and clinical success rate was 91.1% (72/79) and 97.2% (70/72), respectively. Five different techniques were used: 43% (34/79) underwent the balloon-assisted method, 27.9% (22/79) underwent endoscopic ultrasound-guided balloon occluded gastro-jejunostomy bypass, 20.3% (16/79) underwent the direct technique, 6.3% (5/79) underwent the hybrid rendezvous technique, and 2.5% (2/79) underwent natural orifice transluminal endoscopic surgery (NOTES)-assisted procedure. All techniques required an echoendoscope except NOTES. In all, 53.2% (42/79) had non-cautery enhanced Axios stent, 44.3% (35/79) had hot Axios stent, and 2.5% (2/79) had Niti-S spaxus stent. Symptom-recurrence was seen in 2.8%, and 6.3% had a complication (bleeding, abdominal pain or peritonitis). All procedures were performed by experts at centers of excellence with adequate surgical back up.
INTRODUCTION
Gastric outlet obstruction (GOO), afferent limb syndrome (ALS), or biliary obstruction, in patients with altered gastrointestinal (GI) tract anatomy, are the most common presentations in clinical practice which require either surgical and/or endoscopic drainage. Traditionally, endoscopic dilation for benign etiologies and surgery for malignant etiologies has been the standard course of treatment [1]. However, endoscopic dilations require multiple sessions and have variable outcomes, including the risk of perforation [2-4]. Surgical bypass is limited by significant morbidity, prolonged recovery time, and high operative costs [5-7]. In cancer patients with poor surgical tolerance, endoscopic placement of self-expanding metallic stents (SEMS) is an alternative, but is limited by stent obstruction due to tumor ingrowth [1,5-7]. The same may not even be feasible, if the obstruction has led to complete closure of the lumen.
Endoscopic creation of a de-novo anastomosis with the aid of lumen apposing metal stent (LAMS) is an exciting new technique. It is a less invasive alternative to surgery. It can be a therapeutic or palliative intervention depending on the etiology of obstruction. It enables drainage and efficient transit of GI contents (bile, pancreatic enzymes or food contents) by by-passing the site of obstruction which may be due to benign or malignant etiology.
The procedure entails endoscopic localization of two adjacent lumens of the GI tract bypassing the site of obstruction, followed by deployment of LAMS for formation of a fistulous tract. LAMS is a novel saddle shaped, 1-cm long stent, 10 or 15 mm in diameter with a wide flange of 23 and 28 mm on either end. The unique saddle shape of LAMS gives it anti-migratory property and allows it to hold the two GI lumens together. The application of LAMS in this clinical setting is an extension of its known indications, and experience thereof, is still evolving [8-10].
In this article we review the available evidence in form of case reports, case series, retrospective and prospective studies, describing the use of LAMS for de-novo GI anastomosis.
MATERIALS AND METHODS
An extensive English literature search till December 2017 was performed separately by two authors, using PubMed and Google Scholar, to identify peer reviewed original articles using keywords- Lumen apposing metal stent; Gastroenterostomy; Enteroenteric anastomosis. Only human study subject articles in English literature were selected. Additional relevant studies were identified by manually searching the references of pertinent studies.
RESULTS
A preliminary search yielded 14 original studies which included 2 prospectively designed studies [11,12], 3 retrospective studies [13-15], 3 case series [16-18], and 6 case reports [19-24]. On further reading of selected articles, 2 articles were excluded due to duplication of represented population [12,15] and one article was excluded due to lack of data regarding the type of stent used [18]. The etiology and location of obstruction, technical procedure details, stent specifics, outcomes, and complications with their management from each study were reviewed and have been summarized in Tables 1, 2, and 3.
DISCUSSION
Patient characteristics
Demographics
Out of 11 studies, demographic data was reported in 9 studies [11,13,14,16-21,23,24]. The mean age of the cohort was 62.9 years, with the range varying from as young as 34 years to as old as 90 years. The study cohort comprised of 50.7% males (38/75) and 49.3% females (37/75).
Anatomy and indication
Almost half (51.9%) of the cohort had pre-existing altered GI anatomy [11,16,17,19-23].
GOO was the most common indication attributed in 84.8% (67/79) of cases [11,13,14,24]. The second most common indication was ALS, in 11.4% (9/79) cases [16,17,20,21]. The two less common indications were to gain endoscopic access to relieve biliary obstruction (1.3%) [19] and efferent limb obstruction (2.5%) [22,23]. In 74.7% cases, the underlying etiology was malignant [11,13,14,16,17,19-21,24], whereas in the rest it was benign (25.3%) [11,13,17,22,23].
Prior interventions
No data was reported regarding prior interventions in 38% (30/79) of the cohort [14]. Endoscopic ultrasound (EUS)-guided de-novo anastomosis was the preferred as first line treatment in 26.6% (21/79) cases [11,13,16,21-24], and as secondary treatment (after failed primary interventions) in 35.4% (28/79) patients [11,13,17,19,20]. Prior interventions included endoscopic dilation [11], SEMS [11,13], percutaneous endoscopic gastrostomy or jejunostomy [11], nasal-jejunal tube [11], enteroscopy [17,19], endoscopy [20], EUS-guided biliary drainage [19], percutaneous transhepatic biliary drainage [17,19].
Demographics, details of underlying etiology, indication, anatomy, and prior interventions in patients from each individual study have been summarized in Table 1.
Procedure characteristics
Technique
Multiple approaches exist to identify the target small bowel loop.
1. Incomplete occlusion:
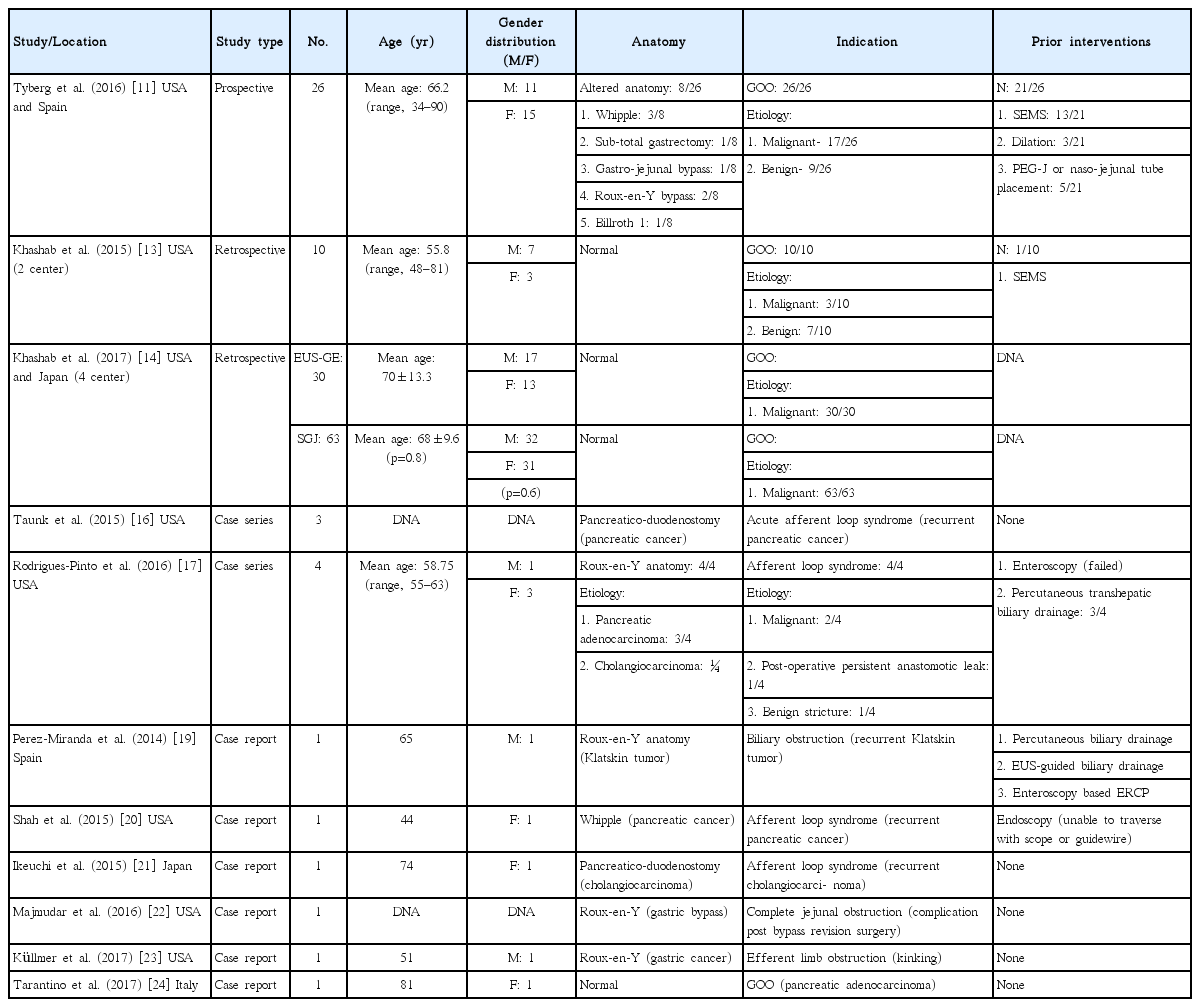
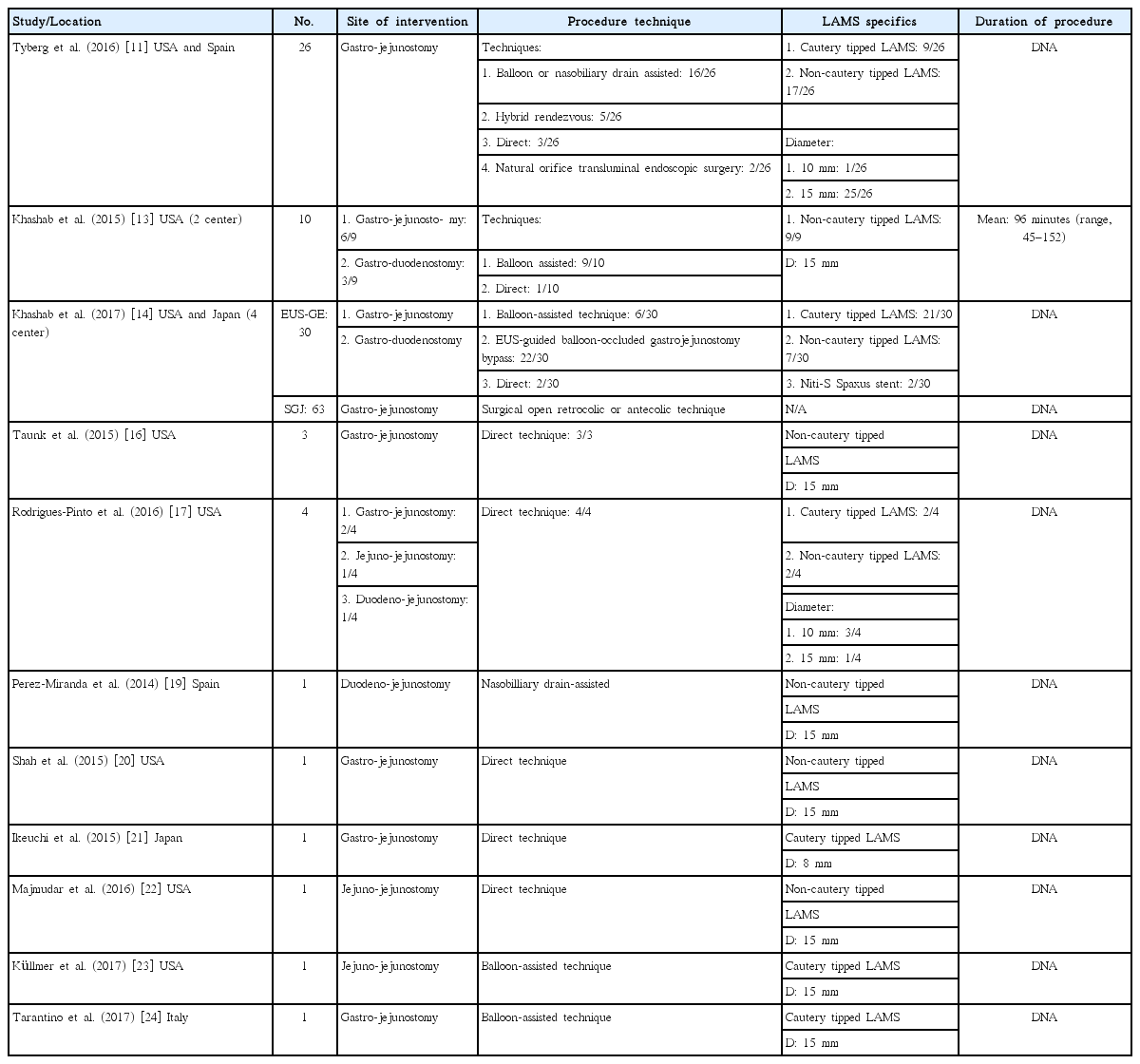
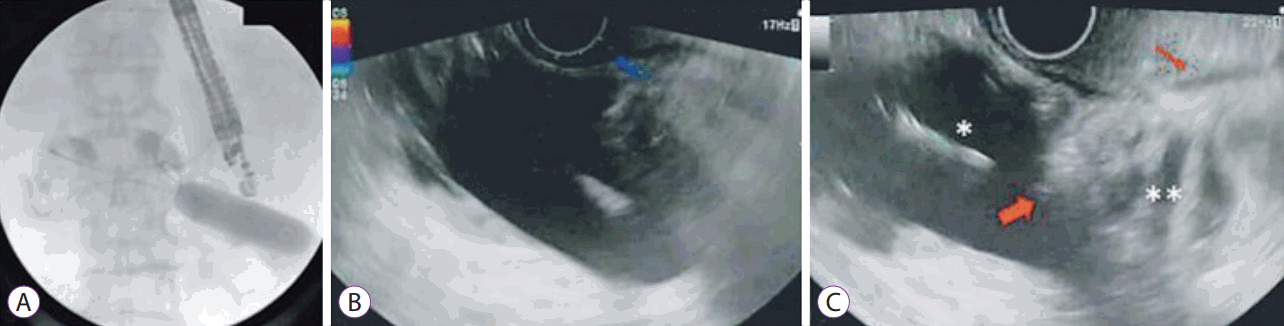
• Balloon or nasobiliary drain assisted: [11,13,14,19,23,24] In this approach, the area of stenosis is traversed either by the endoscope itself or with a guidewire under fluoroscopic guidance (Fig. 1). A balloon dilator or nasobiliary drain is then passed over the guidewire and the balloon is filled with contrast (or the nasobiliary drain is used to insufflate the lumen with water), which can then be localized by an echoendoscope in the stomach (Fig. 2).
• Hybrid rendezvous:11 In this approach, the area of stenosis is traversed by an ultra-thin scope, followed by water insufflation of the distal lumen, which can then be identified by an echoendoscope in the stomach.
• EUS-guided balloon occluded gastro-jejunostomy bypass (EPASS): [14] In this approach, the area of stenosis is first traversed with a guidewire over which a special double balloon enteric tube is passed (Tokyo Medical University Type; Create Medic Co., Yokohama, Japan). The two balloons are then inflated with contrast, followed by saline infusion between the two balloons, which holds the small bowel in close proximity to the gastric lumen and allows easy identification by the echoendoscope.
2. Complete or incomplete occlusion:
• Direct technique: [11,13,14,16,17,19-24] This approach is feasible even in cases where complete lumen obstruction prevents traversing of the site with a scope or a guidewire. The target small bowel loop is identified and confirmed by contrast injection with the help of EUS-guided needle puncture (19 or 22 G).
• Natural orifice transluminal endoscopic surgery (NOTES): [11] This approach is feasible even in cases with complete luminal occlusion. Essentially, in this approach, a full thickness gastric wall incision is made followed by dilation to allow the endoscope to enter the peritoneal cavity, where under direct visualization, a small bowel loop is identified, incised, and a guidewire is placed, over which the stent is deployed. This approach does not require an echoendoscope.
In our study cohort, 43% (34/79) underwent the balloon-assisted method, 27.9% (22/79) underwent EPASS, 20.3% (16/79) underwent the direct technique, 6.3% (5/79) underwent the hybrid rendezvous technique, and 2.5% (2/79) underwent NOTES assisted de-novo entero-enteric or gastro-enteric anastomosis [11,13,14,16,17,19-24]. Chen et al. compared the balloon-assisted and direct EUS-guided techniques and found similar clinical and technical success rates (TSRs), but the mean duration of the procedure was significantly lower in the direct technique (35.1+31.2 min) in contrast to the balloon-assisted technique (89.9+33.3 min) [15].
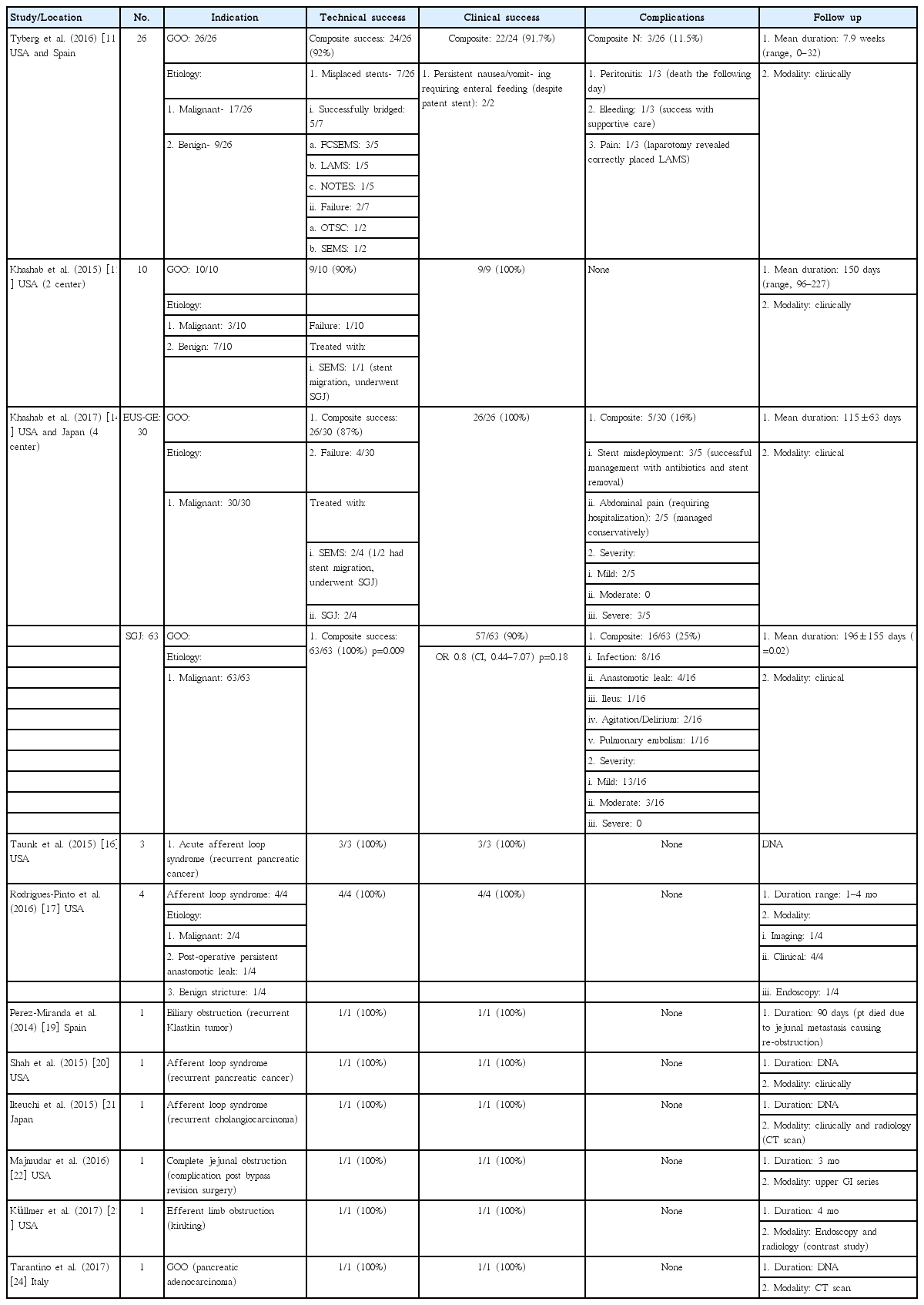
Once the target bowel loop has been identified, the next step depends on whether the intent is to use a cautery enhanced LAMS apparatus or non-cautery enhanced LAMS apparatus. The latter requires puncturing of the target lumen (if containing the contrast filled balloon, will leak into the puncture, thus helping to confirm the lumen location) with an EUS-guided needle (19 or 20 G). This is followed by passage of a guidewire (0.035 inch diameter), balloon dilation of the tract (it is not advisable to dilate more than 4–6 mm, as it may lead to spontaneous misplacement of LAMS), and then deployment of LAMS (distal flange first followed by the proximal flange). Conversely, cautery enhanced LAMS combines all these steps into one step (Fig. 3). LAMS can be deployed under direct EUS guidance alone or in combination with fluoroscopy. Once the LAMS is deployed, a 10 or 15 mm balloon dilator is used to dilate the stent to its intended size. In our study cohort, two types of LAMS were used- Axios (non-cautery enhanced or hot) [11,13,14,16,17,19-24] and Niti-S Spaxus [14]. In all, 53.2% (42/79) had non-cautery enhanced Axios stent, 44.3% (35/79) had hot Axios stent, and 2.5% (2/79) had Niti-S spaxus stent [11,13,14,16,17,19-24]. The stent size and type for each study has been summarized in the Table 2.
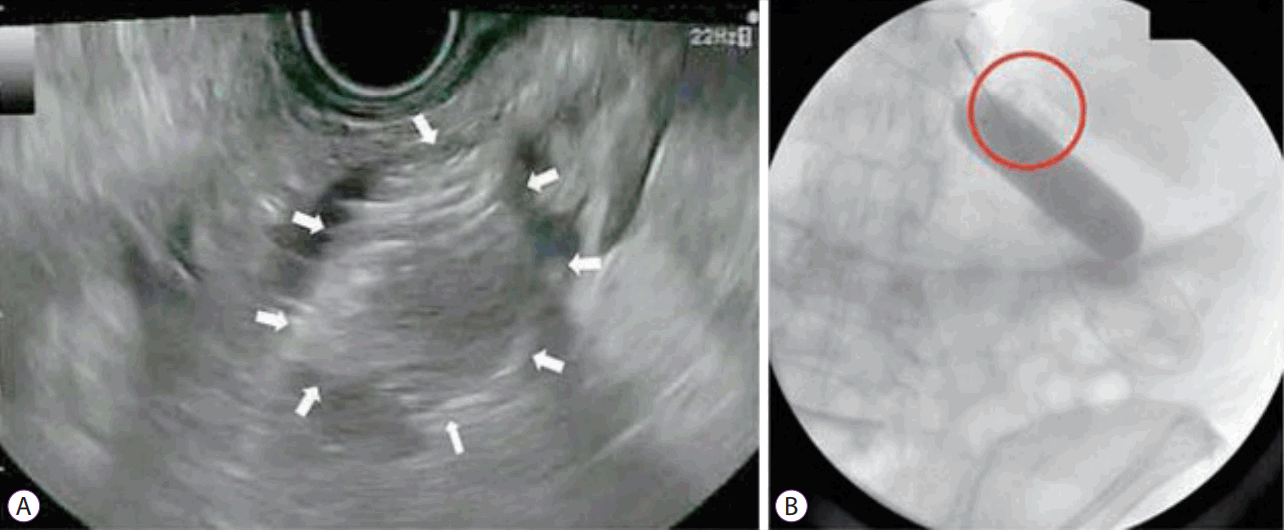
Deployment of the stent. (A) Echoendoscopic view of the released distal flange of the stent (arrows) into the lumen of the jejunal loop. (B) Fluoroscopic view of the fully released stent (circle) and the intact balloon (Re-produced with permission from Thieme publishers).
As the small bowel is mobile, keeping the target loop stationary and in close proximity of the site of intended therapeutic intervention is challenging. To achieve stability, several approaches have been described:
• Glucagon administration to decrease peristaltic movements
• Snare-balloon technique- A snare is attached over the balloon catheter and is used to catch the guidewire passed through the EUS needle. This is followed by application of tension on the snare/balloon apparatus which helps to keep the target bowel loop fixed in place [13]. A modification of this technique was described by Ngamruengphong et al. where both ends of the guidewire are pulled (externally) to achieve the same outcome [18].
In addition, few authors chose to place a pigtail catheter through the LAMS to prevent any recurrence of obstruction [17,20]. This practice has been shown to be useful at alternative sites like choledocho-enterostomy and cholecysto-enterostomy creation with LAMS [8,9].
Outcomes
Follow up
Each author reported a variety of modalities to follow up their patient’s post-procedure: either clinically alone or in combination with radiological studies like- upper GI series, computed tomography scan, or endoscopy. Similarly, a wide variation was noted in the follow up period across the studies as has been summarized in the Table 3.
Technical success and failure
Technical success was defined by adequate placement of LAMS. In total, 79 patients from 11 individual studies underwent EUS-guided de-novo entero-enteric anastomosis. Composite TSR for the cohort was 91.1% (72/79). Individual TSR was 100% for eight studies [16,17,19-24]. For the remaining three, the TSR was 92% (24/26), 90% (9/10), and 87% (26/30), respectively [11,13,14]. Tyberg et al. reported initial misplacement of LAMS in 7 patients (7/26) [11]. The authors were able to successfully bridge the stent in majority of cases (5/7) with the aid of fully covered SEMS (3/5), LAMS (1/5), or NOTES (1/5) [11]. Out of the patients who had technical failure (7/79), 8.9% were managed with SEMS (4/7), over the scope clip (1/7), and surgical gastro-jejunostomy (SGJ- 2/7). Half the patients with SEMS (2/4) experienced migration of the stent and consequently underwent SGJ. In a retrospective comparative study by Khashab et al. the TSR for SGJ was significantly (p<0.009) higher in contrast to that for EUS-guided gastro-enterostomy (EUS-GE) [14]. No significant predictors for technical success were found after adjustments for age, gender, etiology, prior interventions, presence or absence of altered anatomy, and use of LAMS with or without cautery for fistula creation [11].
Clinical success and failure
Clinical success was defined as the ability of the patient to tolerate oral feeds/alleviation of obstructive symptoms after successful placement of LAMS. Out of 72 patients who underwent successful placement of LAMS and creation of new entero-enteric anastomosis, 97.2% (70/72) had clinical success. Individual clinical success rate (CSR) was 100% for all studies [13,14,16,17,19-24] except one [11]. Two patients (2.8%) had persistent nausea/vomiting post procedure requiring enteral feeding despite a patent stent [11].
Khashab et al. reported a higher CSR for EUS-GE group (100%) in contrast to that for the SGJ group (90%), but this difference lacked statistical significance [14]. No significant predictors of clinical success were found after adjustments for age, gender, etiology, presence or absence of altered anatomy, prior interventions, and use of LAMS with or without cautery for fistula creation [11].
Combined technical and clinical success was achieved in 88.6% (70/79) of the study cohorts (n=79).
Recurrence
For the study cohort, over the study specific follow-up period, 97.2% of patients (70/72) remained free of obstructive symptoms. Two patients (2.8%) had recurrence at day 88 (post procedure) and at 3 months (post LAMS removal), respectively [14,17]. The first patient had obstruction secondary to a food bolus and was managed with endoscopic extraction [14]. The second patient had re-stenosis at the site (4 months post procedure) and was successfully managed with re-insertion of LAMS. The underlying etiology was benign [17] and malignant [14], respectively, in these two cases. Compared to EUS-GE cohort (1/26), the SGJ cohort (9/63) had a much higher absolute recurrence although statistical significance was lacking (p=0.081) [14].
Complications
1. Abdominal pain: Only significant pain requiring hospitalization was considered as an adverse event. In all, 3.8% (3/79) had significant abdominal pain. Two of them improved with supportive care alone [14]. One patient underwent diagnostic laparoscopy with no obvious cause and at the discretion of surgeon, underwent SGJ [11].
2. Peritonitis: Overall, 0.7% (1/79) of patients developed this adverse event. The patient had a malignancy, ascites, and peritoneal carcinomatosis, and he died the following day [11].
3. Bleeding: In total, 0.7% (1/79) of patients had this adverse event. This patient was successfully managed with conservative management, including blood transfusion [11].
All outcomes for each individual study have been summarized in Table 3.
CONCLUSIONS
De-novo creation of gastro-enteric or entero-enteric anastomosis with the help of LAMS for patients with GOO, ALS, biliary obstruction among subjects with altered anatomy, and efferent limb obstruction, is a novel, less invasive alternative to surgery. It has been successfully used as the primary (preferred) or secondary (after prior treatment failure) treatment in management of patients with either malignant or benign etiology. The composite technical and CSR for our study cohort was 91.1% and 97.2%, respectively. Only 2.8% had recurrence of symptoms and about 6.3% had some significant complications (bleeding, abdominal pain, or peritonitis). The procedure is new and various techniques have been described and used to achieve the desired outcome. It is advisable that the procedure be done only by experts at centers of excellence with adequate surgical back-up.
Notes
Conflicts of Interest:The authors have no financial conflicts of interest.
Author Contributions
Conceptualization: Deepanshu Jain
Data curation: DJ, Ankit Chhoda, Abhinav Sharma
Formal analysis: DJ
Investigation: DJ
Methodology: DJ
Project administration: DJ, Shashideep Singhal
Resources: DJ, SS
Supervision: SS
Writing-original draft: AC, AS
Writing-review&editing: DJ, SS