INTRODUCTION
Endoscopic procedures are considered standard modalities for diagnosing and treating several disorders of the digestive tract. Evaluation of the risks involved in performing upper and lower gastrointestinal endoscopy is an important concern for gastroenterologists. Endoscopic complications are classified into 4 grades depending on the need for hospitalization and the extent of bleeding. Events that require 1 to 3 days of hospitalization are graded as mild, while those requiring 4 to 9 days are graded as moderate. Severe events are defined as those that require more than 10 days in the hospital, admission under intensive care, or where surgery must be performed. Endoscopic procedure-related events that lead to death attributable are classified as fatal [1]. Colonoscopy is generally well-tolerated as a procedure. Transient gastrointestinal symptoms, such as abdominal pain, bloating, and nausea, and side effects related to sedation and analgesia, are reported by about 33% of patients [2,3]. Major complications related to colonoscopy include colonic perforation, post-polypectomy bleeding, post-polypectomy syndrome, and rarely, splenic rupture, acute appendicitis, and diverticulitis. Acute pancreatitis is a complication that is associated with endoscopic procedures involving ampullary cannulation [4]. However, case reports of pancreatitis following routine esophagogastroduodenoscopy and colonoscopy suggest that ampullary cannulation is not a requirement for the development of acute pancreatitis [5-11]. Colonoscopy-induced pancreatitis is an extremely rare phenomenon that can be easily missed, which leads to delays in diagnosis and treatment. Up to the time of writing, there have only been a few reported cases in literature regarding the development of acute pancreatitis after colonoscopy [6-11]. We describe a probable case of acute pancreatitis caused by a difficult colonoscopy at our center.
CASE REPORT
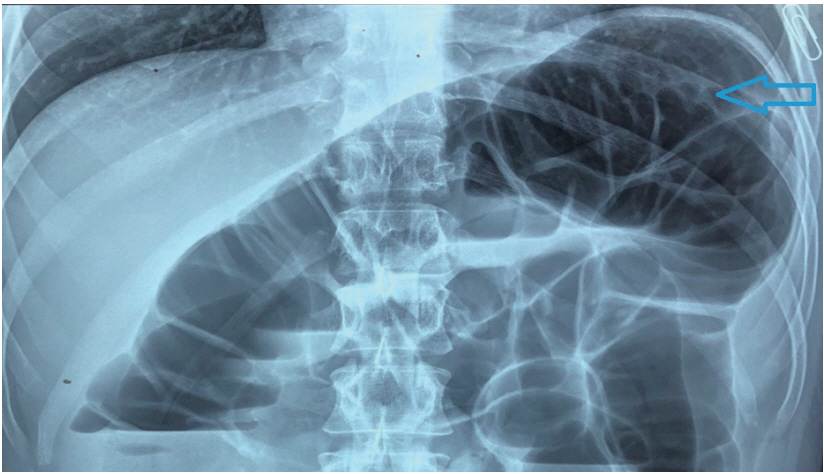
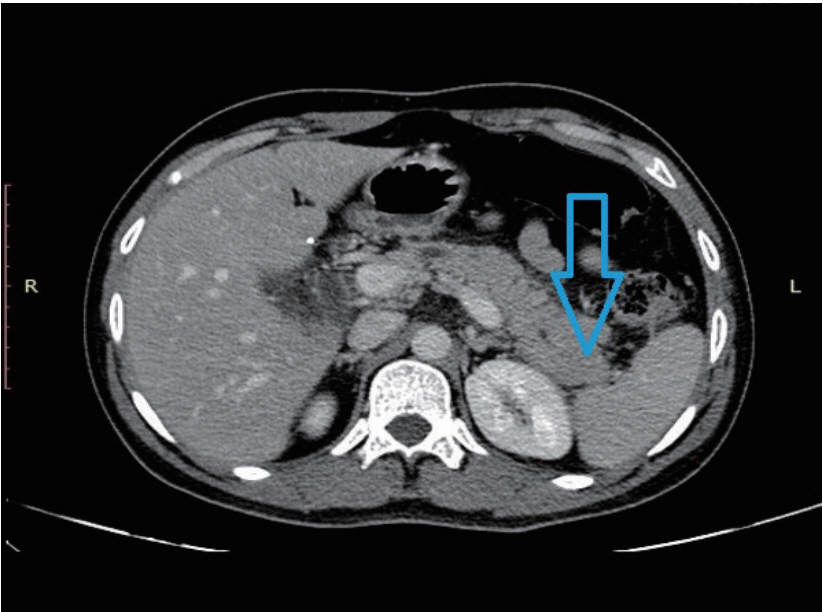
A 54-year-old lean, asymptomatic male underwent elective colonoscopy at our center under sedation due to a family history of colon cancer in his father. The patient is reportedly a nonalcoholic and is not addicted to tobacco in any form. He had no major medical illness in the past and was not on any medications. The general and systemic physical examination was normal. The patient underwent split-dose bowel preparation using polyethylene glycol in two liters of water and kept on a clear liquid diet the day prior to the procedure. Propofol and midazolam were used as premedication. Colonoscopy was then performed with a Boston Bowel Preparation Scale of 6. Air insufflation was used during the procedure due to the unavailability of carbon dioxide. The colonoscope was navigated up to the terminal ileum with the formation of an alpha loop in the sigmoid colon. Due to tight angulation at the splenic flexure, the scope was maneuvered with the aid of external abdominal pressure and by adjusting the patient to the left lateral position. The total cecal intubation time was 12 minutes. Colonoscopy findings showed two sessile polyps, both measuring 5 mm, in the rectum and sigmoid colon; these were removed with the use of cold forceps. Electrocautery was not used during the procedure. Two hours after the procedure, the patient developed acute abdominal pain and an episode of non-bilious, non-bloody emesis. He had a pulse rate of 90 beats per minute, a blood pressure of 130/90 mm Hg, and a respiratory rate of 26 breaths per minute. On physical examination, the patient had epigastric tenderness without signs of peritonitis. There was no hepatomegaly or splenomegaly on palpation. Laboratory tests revealed a hemoglobin level of 14.2 g/dL, a total leucocyte count of 14,500/uL, serum amylase levels of 1,842 U/L, and serum lipase levels of 2,460 U/L. The patientтs C reactive protein was at 28 mg/L (Normal <5 mg/L). Serum electrolytes were normal, as were his liver function tests, serum calcium levels, parathyroid hormone levels, and lipid profile. A erect abdominal X-ray did not reveal any evidence of free air but demonstrated a distention of the descending colon, splenic flexure, and transverse colon, possibly due to air insufflation during the procedure (Fig. 1). Computed tomography (CT) scan of the abdomen revealed edema of the body and tail of the pancreas with mild peripancreatic stranding; these findings are consistent with acute pancreatitis (Fig. 2). There was no evidence of calculi in the gall bladder or common bile duct on imaging. The patient was conservatively managed with hydration, bowel rest, and analgesics. The symptoms improved over five days and he was eventually shifted to a regular diet. The patient was discharged after 7 days.
DISCUSSION
Acute pancreatitis, while being a well-known complication of endoscopic procedures like endoscopic retrograde cholangiopancreatography and double-balloon enteroscopy, is not commonly associated with esophagogastroduodenoscopy and colonoscopy [12,13]. However, the occurrence of low-grade pancreatitis after esophagogastroduodenoscopy or colonoscopy may be more common than previously reported. Blackwood et al. and Kobayashi et al. reported hyperamylasuria and hyperamylasemia in patients who underwent routine diagnostic endoscopy without any evidence of symptomatic pancreatitis [14,15]. The patientтs history, the clinicianтs examination, and the index of suspicion determine the pretest probability of acute pancreatitis after an endoscopic procedure. The presence of acute epigastric pain, the elevation of lipase/amylase levels more than three times the upper limit of normal, and characteristic findings in contrast-enhanced CT scan are diagnostic of pancreatitis and were met by the patient in this case.
While it is possible that the onset of pancreatitis after the patientтs procedure was coincidental, the temporal profile of the symptoms suggests a causal relation. Other possible etiologies were explored given the innocuous nature of colonoscopy, but the patient had none of the customary risk factors for the development of acute pancreatitis. The patient did not have any history of addiction nor was there any evidence of intake of any drugs that can cause pancreatitis. The patient denied any history of abdominal trauma prior to the procedure. His serum calcium levels, parathyroid hormone levels, and lipid profile were all normal. Furthermore, there was no history of cholelithiasis, choledocholithiasis, or previous episodes of pancreatitis.
While the pathophysiology of post-colonoscopy acute pancreatitis is not postulated in literature, there are papers that explain the mechanism for the development of pancreatitis after esophagogastroduodenoscopy and double-balloon enteroscopy [12,13]. During antegrade double-balloon enteroscopy, one balloon may be inflated beyond the ampulla while the other balloon remains in the duodenal bulb leading to the creation of a closed segment. Duodenal hypertension then consequently develops and exerts direct pressure on the pancreas or the pancreatic duct thereby leading to pancreatitis [13]. Reflux of duodenal juices into the pancreatic duct due to duodenal overdistension may also explain the development of pancreatitis after esophagogastroduodenoscopy [16].
The mechanism for the development of acute pancreatitis after colonoscopy is uncertain. In our case, repeated withdrawal and maneuvering during the procedure, in the form of abdominal compression, difficult intubation, looping of the instrument, entry into the terminal ileum, and formation of an alpha loop, may have caused mechanical and pressure trauma to the splenic flexure and ultimately precipitated the development of pancreatitis just after the procedure. In this case, acute pancreatitis was localized to the body and tail of the pancreas. It is hypothesized that, due to the anatomic proximity of the pancreatic tail to the splenic flexure, manipulation of the colon causes trauma to the pancreas. Additionally, the use of electrocautery near the splenic flexure is also known to cause transmural burns. These mechanisms can cause trauma and irritation to the pancreatic tail and precipitate an inflammatory response leading to acute pancreatitis. However, there was no use of electrocautery in this case.
There are few case reports regarding colonoscopy-induced pancreatitis in literature. In one case report, Ko et al. reported a technically-challenging colonoscopy, which presented as difficulty in navigating the splenic flexure, in a patient who presented with iron deficiency anemia and colonic polyp [6]. Difficulty in navigating the splenic flexure was also reported by Thomas et al. and Shekhar et al. in patients whose indications for colonoscopy were diarrhea and cancer surveillance, respectively [7,9]. However, some patients who developed pancreatitis after colonoscopy did not necessarily experience a difficult procedure [8,10]. Post-colonoscopy pancreatitis was localized to the tail of the pancreas in reports by Thomas et al. and Khashram et al. [7,10]. A case of recurrent pancreatitis in a patient with a history of multiple surgeries, inflammatory bowel disease, and a previous episode of pancreatitis has also been reported [11]. Raper reported a case of acute pancreatitis secondary to exercise-induced dehydration and reviewed its pathogenesis [17]. Our patient had normal vital signs, did not show any signs of dehydration secondary to polyethylene glycol use during colonoscopy preparation, and had adequate hydration prior to the procedure. Both the capillary refill time and serum electrolyte levels were normal in our patient. Abdominal pain in patients after colonoscopy warrants evaluation to rule out perforation and ileus. After excluding the more common causes of abdominal pain, acute pancreatitis should be considered as a differential diagnosis. While obtaining informed consent, the potential for post-colonoscopy pancreatitis should be explained. For the optimal management of these patients, identification of potential complications during endoscopy and adequate knowledge of appropriate therapies are of paramount importance.