Histological Architecture of Gastric Epithelial Neoplasias That Showed Absent Microsurface Patterns, Visualized by Magnifying Endoscopy with Narrow-Band Imaging
Article information
Abstract
Background/Aims
The objective of this study was to elucidate the histological structure of the absent microsurface patterns (MSPs) that were visualized by magnifying endoscopy with narrow-band imaging (M-NBI).
Methods
The study included consecutive gastric epithelial neoplasias for which M-NBI findings and histological findings could be compared on a one-to-one basis. The lesions were classified as absent MSPs and present MSPs based on the findings obtained using M-NBI. Of the histopathological findings for each lesion that corresponded to M-NBI findings, crypt opening densities, crypt lengths, crypt opening diameters, intercrypt distances, and crypt angles were measured and compared.
Results
Thirty-six lesions were included in the analysis; of these, 17 lesions exhibited absent MSP and 19 lesions exhibited present MSP. Comparing the histological measurements for absent MSPs vs. present MSPs, median crypt opening density was 0.9 crypt openings/mm vs. 4.8 crypt openings/mm (p<0.001), respectively. The median crypt length, median crypt opening diameter, median intercrypt distance, and median crypt angle were 80.0 μm vs. 160 μm (p<0.001), 40.0 μm vs. 44.2 μm (p=0.09), 572.5 μm vs. 166.7 μm (p<0.001), and 21.6 degrees vs. 15.5 degrees (p<0.001), respectively.
Conclusions
Histological findings showed that lesions exhibiting absent MSPs had lower crypt opening density, shorter crypt length, greater intercrypt distance, and larger crypt angle.
INTRODUCTION
In recent years, magnifying endoscopy with narrow-band imaging (M-NBI) has permitted the diagnosis of small, flat early gastric cancers, which are difficult to diagnose using conventional white-light endoscopy [1]. In addition, the results of numerous exploratory studies examining the relationship between magnifying endoscopy findings and histological findings have been reported [2-9]. However, the viewing conditions in these investigations, such as the degree of magnification obtained by magnifying endoscopy, have not been uniform, and no studies have reported on a one-to-one correspondence between the site visualized by magnifying endoscopy and the histopathological findings. Uchita et al. reported that the diagnostic accuracy of magnifying endoscopy differs according to the viewing conditions [10], and that it is ideal to establish uniform viewing conditions at maximum magnification. Major advances in endoscopic diagnosis have been achieved because the morphological features have been confirmed histologically. With magnifying endoscopy, however, the region observed is more minute, and a one-to-one correspondence is therefore required when examining the histological findings for the observed sites. Consequently, Yao et al. reported that in order to match resected gastric epithelial tumor specimens to endoscopy images, it is important to record images of indices that provide orientation and markings that serve as landmarks before the resection is performed [11]. In addition, Doyama et al. described a method of marking that indicates the region of interest on a lesion observed by magnifying endoscopy [12].
Furthermore, Kanesaka et al. reported a significantly higher proportion of undifferentiated-type adenocarcinomas when absent microsurface patterns (MSPs) account for 50% or more of the regions of early gastric cancers, as observed by magnifying endoscopy [4]. This suggests that it is possible to diagnose undifferentiated-type adenocarcinomas endoscopically before surgery. However, it is not known why these cancers have absent MSPs.
Given this background, the present study aimed to elucidate the mechanism that results in absent MSPs, as observed by magnifying endoscopy. To accomplish this, histological findings that reliably matched the sites of the findings obtained by M-NBI under uniform viewing conditions, were compared with those findings on a one-to-one basis, and the differences in the histological findings between lesions that exhibit absent MSPs and those that exhibit present MSPs were examined.
MATERIALS AND METHODS
The study was a single-center, retrospective, observational study and was conducted after being approved by the ethics committee of Fukuoka University (R18-050).
Inclusion criteria
Of the early gastric cancer lesions treated at Fukuoka University Chikushi Hospital between August 2015 and January 2018, the consecutive lesions for which all of the following conditions were fulfilled were included in the study: the lesion was a gastric epithelial neoplastic lesion (carcinoma and adenoma), where two marks demarcating the region of interest were added and could be identified (Fig. 1A). The area between the two marks was observed by magnifying endoscopy at maximum magnification (Fig. 1B, C), and a tissue section was prepared from a specimen site between the two marks (Fig. 1D-F).
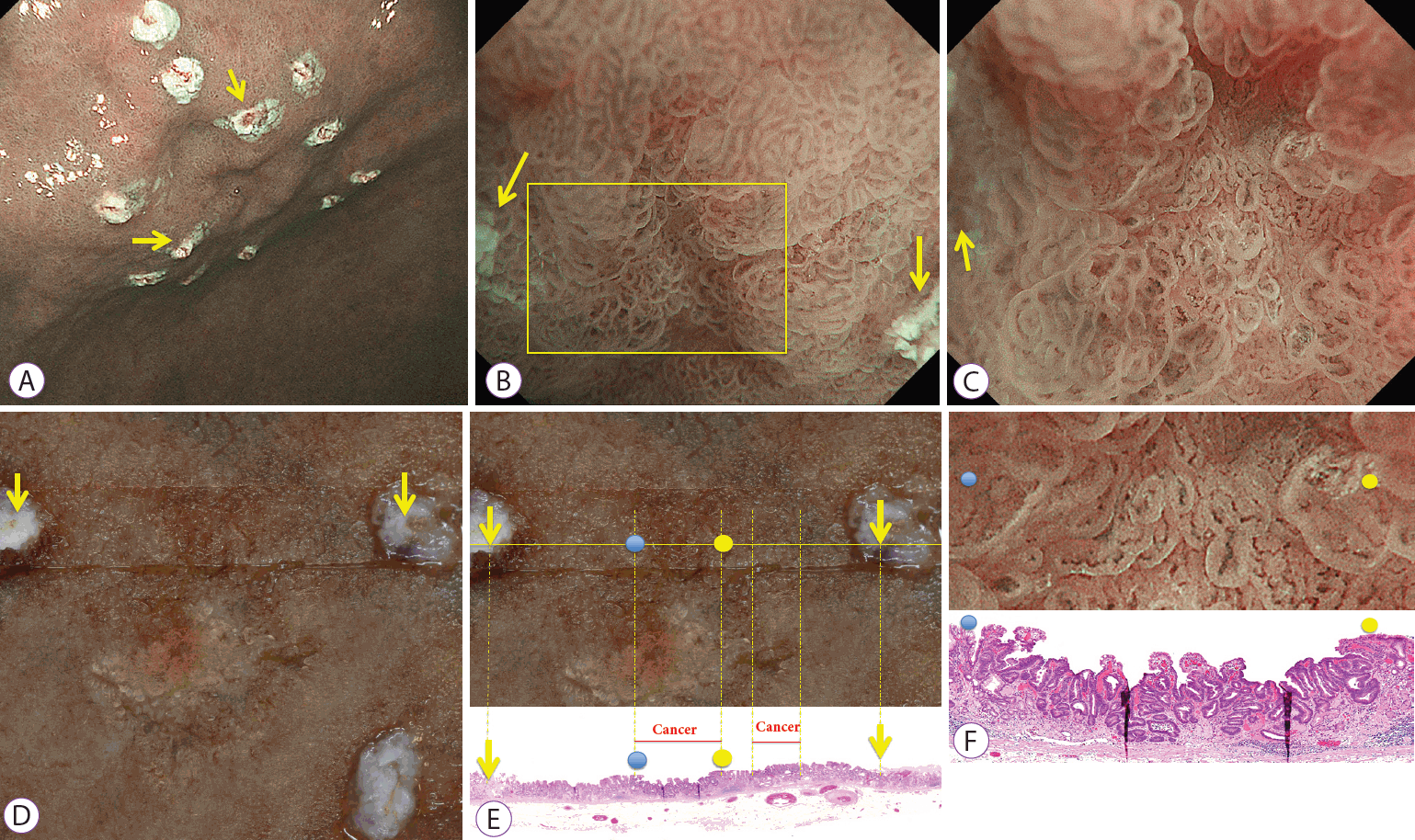
Correspondence between the regions of interest in images obtained by magnifying endoscopy with narrow-band imaging (M-NBI) and matching histological findings. (A) Image obtained by M-NBI. In addition to marks added before endoscopic submucosal dissection (ESD) to indicate the extent of the resection, M-NBI was used to place marks at two points on either side of the lesion. (B) M-NBI: weak magnification. Magnified observation of the area between the two marks was performed by M-NBI (the arrows indicate the marks). (C) M-NBI: maximum magnification. Magnified observation was performed for the area enclosed by the square shown in (B), and the recorded endoscopic findings were used (arrows indicate marks). (D) Formalin-fixed specimen after ESD. After the lesion was resected by ESD, stretched, and fixed in place on a rubber plate with pins, the two marks made before ESD were identified. A cut was made between the two marks, sections were prepared, and hematoxylin-eosin staining was performed. (E) Post-ESD formalin-fixed specimens (top) matched with histological findings (bottom). Marks on the prepared sections were first identified, and histological findings for the area between the marks were observed (arrows indicate marks). Blue and yellow circles on the resected specimen at the top correspond, respectively, to the blue and yellow circles in the histological findings on the bottom. (F) Correspondence between M-NBI (maximum magnification, top) and histological findings (bottom). Histological findings corresponding to the region of interest in the image obtained by M-NBI were identified using the two marks from before the resection (C) and the two marks in the histological findings (E). Blue and yellow circles on the image obtained by M-NBI at the top correspond, respectively, to the blue and yellow circles in the histological findings on the bottom.
Exclusion criteria
Lesions for which magnifying endoscopic imaging was not performed at maximum magnification and those that could not be examined histopathologically due to factors such as severe tissue crushing were excluded.
Endoscopic procedures
After obtaining written informed consent from the patient, endoscopy was performed by three experienced endoscopists (KY, TN, and MM). For all lesions, M-NBI was performed using a magnifying endoscope (GIF-Q290Z or GIF-Q240Z; Olympus Medical System Co., Tokyo, Japan) and an endoscopy system (EVIS LUCERA ELITE; Olympus Medical System) with a black soft hood (MAJ1989; Olympus Medical System) attached to the endoscope tip. The tip was brought to a distance of 2 mm from the mucosa; this distance was maintained, and images obtained at maximum magnification were used. Details of the procedure were described previously [13].
Determining MSP using M-NBI: definition of absent MSPs vs. present MSPs
Lesions included in the study were classified as belonging to either the present MSP group (Fig. 2A) or absent MSP group (Fig. 2B) based on the surface microstructure in the region of interest, as observed by magnifying endoscopy. Present MSP was defined as such when the marginal crypt epithelium could be observed in three-quarters or more of the region of interest. Absent MSP was defined as such when the marginal crypt epithelium could be observed in only one-quarter or less of the region of interest. The MSP was determined by an experienced endoscopist (KY) who was blinded to the histological findings.
Histopathological assessment
The following histopathological measurements were performed for the histopathological findings corresponding to the region of interest, as observed by magnifying endoscopy: (1) crypt opening density, determined by dividing the number of crypt openings by the distance examined (Fig. 3A); (2) crypt length, the distance from the center of the crypt opening to the deepest part of the crypt (Fig. 3B); (3) crypt opening diameter, the distance across the crypt opening (Fig. 3C); (4) intercrypt distance, the distance between crypts (Fig. 3D); and (5) crypt angle, the angle between a line connecting the center of the crypt opening to the deepest part of the crypt, and a line drawn vertically from the crypt opening to the muscularis mucosae (Fig. 3E). The histopathological measurements were performed by an independent single pathologist (KC) who was blinded to the endoscopic findings. The tissue-type classification was performed according to the Japanese Classification of Gastric Carcinomas (14th edition) [14].
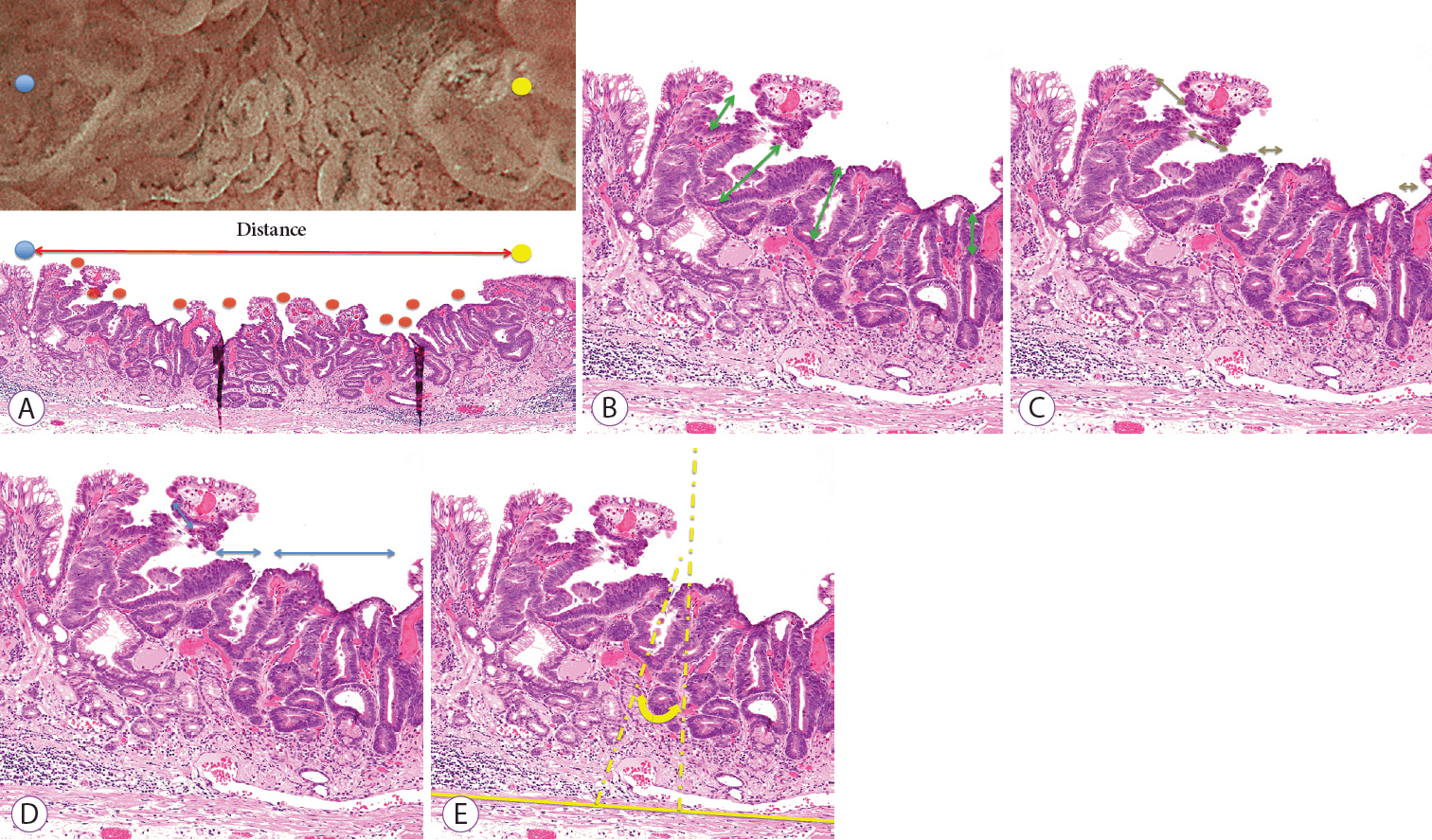
Histological measurements. (A) Crypt openings density = number of crypt openings / examined distance. The red circles indicate crypt openings. (B) Crypt length (double-headed arrow). (C) Crypt opening diameter (double-headed arrow). (D) Intercrypt distance (double-headed arrow). (E) Crypt angle (double-headed arrow).
Endpoints
The following were determined:
1) Differences in histological measurements between the absent MSP and present MSP groups.
2) Differences in clinical pathology features between the absent MSP and present MSP groups.
Statistical analysis
Between-group statistical tests of the mean and median values for the continuous variables were performed using the Student’s t-test and Mann–Whitney U test, respectively. Between-group statistical tests of frequencies were performed using Fisher’s exact test and linear by linear association test. The Statistical Package for Social Science (SPSS) for Windows ver. 22 (IBM SPSS Co., Armok, NY, USA) was used for the statistical analyses.
RESULTS
Thirty-seven gastric epithelial neoplastic lesions fulfilled the inclusion criteria and did not meet any of the exclusion criteria followed in the study. One lesion was then excluded due to tissue crushing. Consequently, 36 lesions (32 adenocarcinomas and 4 adenomas) were included in the analysis.
The findings obtained by M-NBI showed that 17 lesions (42.7%) exhibited absent MSPs and 19 (52.8%) exhibited present MSPs.
Histological measurements for absent MSP and present MSP groups
Three typical lesions from the absent MSP group and one from the present MSP group are shown, along with their measurement values, in Fig. 4A-C.
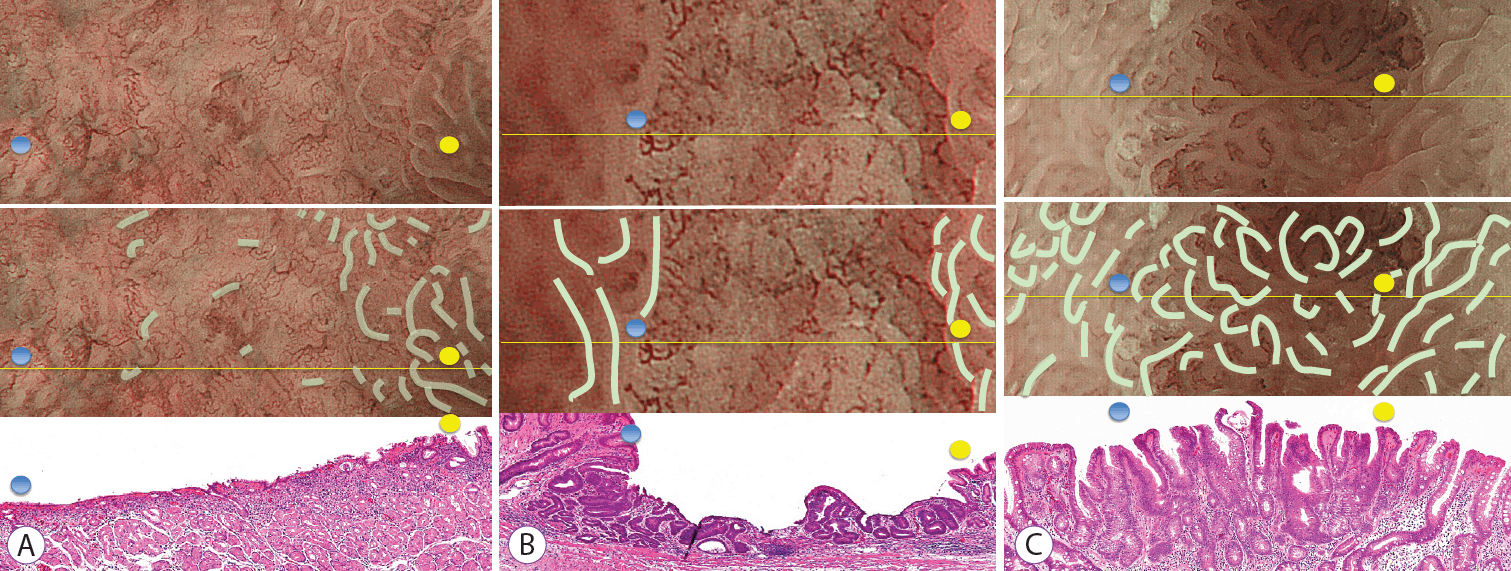
Typical examples and histological measurements (mean±standard deviation for crypt length, crypt opening diameter, intercrypt distance, and crypt angle) for the absent microsurface pattern (MSP) and present MSP groups. (A) Example of early gastric cancer showing an absent MSP using magnifying endoscopy with narrow-band imaging (M-NBI). Histological findings indicated moderately to poorly differentiated adenocarcinomas. It’s crypt opening density was 2 crypt openings/mm, crypt length was 80±87.5 μm, crypt opening diameter was 35.0±8.7 μm, intercrypt distance was 256.7±140.1 μm, and crypt angle was 15.5±20.9 degrees. (B) Example of early gastric cancer showing an absent MSP using M-NBI. Histological findings showed a well-differentiated adenocarcinoma. It’s crypt opening density was 1.71 crypt openings/mm, crypt length was 100±25 μm, crypt opening diameter was 56.7±5.8 μm, intercrypt distance was 350±282.3 μm, and crypt angle was 27.3±19.6 degrees. (C) Example of early gastric cancer showing a present MSP. Histological findings demonstrated a well-differentiated adenocarcinoma. It’s crypt opening density was 6.28 crypt openings/mm, crypt length was 224.5±101.3 μm, crypt opening diameter was 40.9±18.7 μm, intercrypt distance was 120±48.5 μm, and crypt angle was 12.5±12.5 degrees.
The results of a comparison of the histological measurements in the absent MSP and present MSP groups were as follows. (1) The median (range) crypt opening density was 0.9 crypt openings/mm (0.0 to 3.0 crypt openings/mm) vs. 4.8 crypt openings/mm (1.88 to 9.33 crypt openings/mm), respectively (p<0.001). (2) The median crypt length (range) was 80.0 μm (0.0 to 140.0 μm) vs. 160 μm (55.7 to 415.3 μm), respectively (p<0.001). (3) The median crypt opening diameter (range) was 40.0 μm (0.0 to 80.0 μm) vs. 44.2 μm (22.5 to 100.0 μm), respectively (p=0.09). (4) The median intercrypt distance (range) was 572.5 μm (190.0 to 300.0 μm) vs. 166.7 μm (90.0 to 428.3 μm), respectively (p<0.001). (5) The median crypt angle (range) was 21.6 degrees (16.6 to 90.0 degrees) vs. 15.5 μm (7.3 to 28.7 degrees), respectively (p<0.001).
Clinical pathology features included in the analysis
In terms of age, sex, location, macroscopic type, and histological type, the absent MSP and present MSP groups differed only with respect to location, with the lesions tending to be present at lower locations in the present MSP group (Table 1). When examining the histological types, the two groups were compared with respect to lesions that included undifferentiatedtype adenocarcinomas (n=10) and other lesions excluding adenomas (n=22), the ratio that included undifferentiated-type adenocarcinomas was significantly higher in the absent MSP group than in the present MSP group (p=0.007; Table 2).
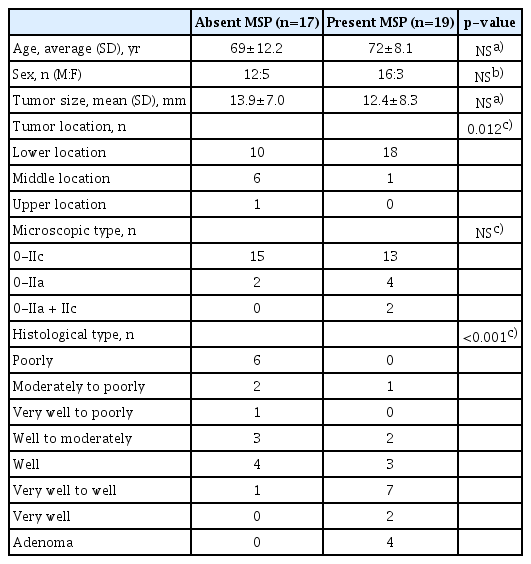
Comparison of Clinical Pathology Features for Absent Microsurface Pattern and Present Microsurface Pattern Groups
DISCUSSION
The histological findings in this study showed that lesions exhibiting absent MSPs had lower crypt opening density, shorter crypt length, greater intercrypt distance, and larger crypt angle. The results obtained in this study represent the first reported results that describe the composition of the histopathological structure of epithelial neoplasms that exhibit an absent MSP, as determined by a one-to-one correspondence between magnifying endoscopy images obtained under constant viewing conditions and histopathological findings. Yao inferred that the mechanism by which the marginal crypt epithelium, a surface microstructure, is visualized involves backward scattering of projected light by the vertically arranged marginal crypt epithelium, with vertical accumulation of the backward scattering resulting in visualization of the whitish, semi-transparent marginal crypt epithelium (Fig. 5A) [15]. Considering the results of the present study in light of this hypothesis, it was inferred that the marginal crypt epithelium is not visualized due to a low crypt opening density or a large intercrypt distance, resulting in a reduced number of crypts that cause backward scattering. This prevents visualization of the marginal crypt epithelium, and thus results in an absent MSP (Fig. 5B). In addition, if the crypt length is short or the crypt angle large, the vertical backward scattering accumulation may be inadequate, preventing visualization of the marginal crypt epithelium (Fig. 5C, D).
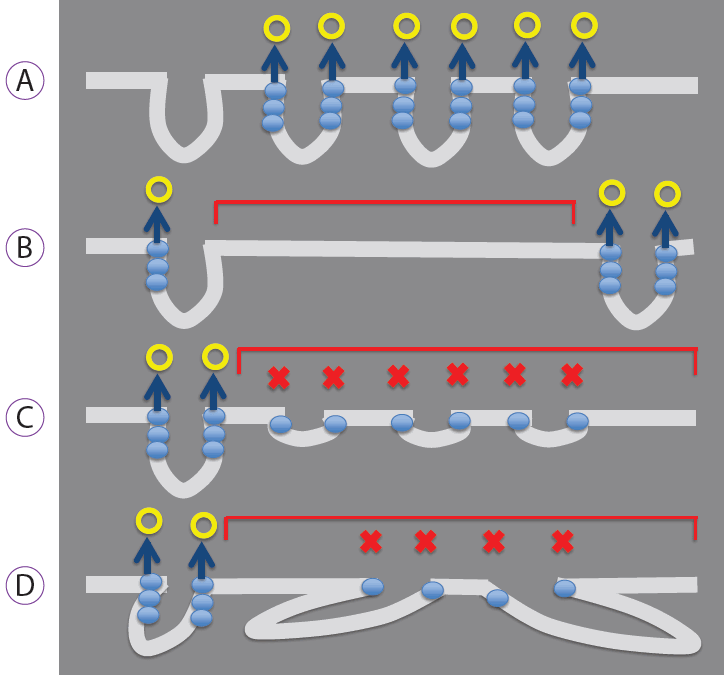
Mechanisms by which the marginal crypt epithelium is visualized and not visualized. (A) Mechanism of visualization. Backward scattering caused by the marginal crypt epithelium (blue circle) is vertically integrated (blue arrow), and the epithelium is visualized (yellow circle). (B-D) Mechanisms that make visualization difficult. (B) If the crypt opening density is low or the intercrypt distance large, the number of crypts that cause backward scattering (blue circle) is reduced, resulting in the absent microsurface pattern (MSP; red square brackets). (C) If the crypt length is short or (D) the crypt angle is large (i.e., crypts run obliquely), the backward scatter (blue circle) does not integrate vertically. Consequently, the epithelium is not visualized as a white, belt-like structure (marginal crypt epithelium), resulting in the absent MSP (red square brackets).
Yagi et al. examined crypt depth and intercrypt distance after dividing lesions into three groups: (1) in which the marginal crypt epithelium could be observed, (2) in which it was difficult to observe, and (3) in which it could not be observed [16]. They reported that for marginal crypt epithelium that could not be observed or was difficult to observe, the intercrypt distance was short and crypt depth was shallow [16]. Although our results were similar for crypt depth, they differed for intercrypt distance. This difference may be due to differences in viewing conditions, the criteria for inclusion in the MSP group, and the lesions included in the study. If the magnification is low, the surface microstructure of some lesions is difficult to visualize when the crypts are densely arranged. However, we observed all lesions under the same condition of maximum magnification. Consequently, the marginal crypt epithelium was visualized, even in lesions with a high crypt density, and they were therefore assessed as a present MSP. Investigations that use images obtained under uniform viewing conditions, as in the present study, will likely provide results of greater reproducibility.
Kanesaka et al. reported a significantly higher proportion of undifferentiated carcinomas when 50% or more of the region of a lesion consisted of an absent MSP [4]. In this study, the frequency of undifferentiated-type carcinomas was significantly higher in the absent MSP group than in the present MSP group. However, differentiated-type carcinomas in the absent MSP group were found in eight lesions (47.1%). A potential reason for this unexpected increase in proportion is that most differentiated-type carcinomas exhibiting an absent MSP may have been included because only certain parts of the lesions were extracted and studied. Therefore, Kanesaka et al. [4] advocates that histological type diagnoses be performed on lesions where absent MSPs account for 50% or more of the entire lesion.
In contrast, a case of undifferentiated-type carcinoma was observed in the present MSP group. As reported by Phalanusitthepha et al., the stretch sign, an expansion of the area between crypts, is a finding specific to magnifying endoscopy observations in early-stage signet-ring cell carcinoma [17], and lesions that do not exhibit absent MSPs may be included among undifferentiated-type tumors that are 2 cm or less in size, for which endoscopic submucosal dissection is indicated. In the present study as well, the findings obtained by M-NBI for small tumors associated with signet ring cells or undifferentiated-type cancer, primarily in the middle layer of the mucosa, were described as indicative of present MSP.
Consequently, a limitation of this study was that the magnifying endoscopy images were obtained at a precise maximum magnification, and lesions for which a reliable comparative examination could be performed were sampled. Consequently, the large number of small carcinomas with relatively small lesions may have resulted from a bias in lesion selection. This study was a retrospective pilot study. Therefore, to resolve these problems, we are planning a prospective study that will include consecutive cases.
In conclusion, we demonstrated that the histological structure of lesions that exhibit absent MSP can, to a certain extent, be estimated. However, the parts observed by magnifying endoscopy are changes near the surface epithelium, and deeper changes cannot be seen directly. When an absent MSP site is seen, inferring the presence of a histological structure with features such as a low crypt opening density, short crypt length, greater intercrypt distance, and a large crypt angle is useful for accurate histological diagnosis by magnification endoscopy.
Notes
Conflicts of Interest: The authors have no potential conflicts of interest.
Funding: This study was funded by Central Research Institute for Endoscopy, Fukuoka University.
Acknowledgements
We would like to thank Dr Hisatomi Arima (Department of Preventive Medicine and Public Health, Fukuoka University, Fukuoka, Japan) for his constructive discussion regarding statistical analysis.
Notes
Author Contributions
Conceptualization: Kenshi Yao
Data curation: Kenta Chuman
Formal analysis: KC
Investigation: KC, Takashi Nagahama, Masaki Miyaoka, Haruhiko Takahashi, Kentaro Imamura, Rino Hasegawa, Toshiharu Ueki, Hiroshi Tanabe, Seiji Haraoka, Akinori Iwashita
Project administration: KY
Supervision: KY
Visualization: Takao Kanemitsu
Writing-original draft: KC
Writing-review&editing: KY


