Endoscopic Ultrasound-Guided Fine Needle Aspiration Using a 22-G Needle for Hepatic Lesions: Single-Center Experience
Article information
Abstract
Background/Aims
Endoscopic ultrasound-guided fine needle aspiration (EUS-FNA) has been accepted as a reliable tool in diagnosing and staging intra-abdominal tumors. In this study, we aimed to investigate the performance of EUS-FNA in the evaluation of liver masses and its impact on patient management and procedure-related complications retrospectively.
Methods
Data of patients who underwent EUS-FNA biopsies due to liver masses between November 2017 and July 2018 were retrieved retrospectively. Biopsies were performed using 22-G needles. The demographics, EUS-FNA results, sensitivity and specificity of the procedure, negative predictive value, positive predictive value, and specimen sufficiency rates were assessed.
Results
A total of 25 patients (10 females) were included in the study. The mean age was 62.73±15.2 years. The mean size of the masses was 34.50±16.04 mm. The technical success rate was 88%. During the EUS-FNA procedure, each patient had only one pass with 94.45% of aspirate sufficiency rate and 86.3% of biopsy sufficiency rate. The diagnostic accuracy rate was 86.3%. There were no complications.
Conclusions
For the evaluation of liver masses, EUS-FNA using a 22-G needle with even one pass had high aspiration and biopsy success rates accompanied with high diagnostic accuracy rates.
INTRODUCTION
The diagnosis of hepatic masses is important in clinical decision-making and typically established through percutaneous pathways with ultrasound and/or computed tomography (CT)-guided tissue biopsy for histopathological examination [1]. However, this procedure becomes challenging or impossible in small-sized lesions because of the difficulty in reaching the lesions by percutaneous means and biliary or vascular structures intervening on the biopsy line. In these cases, endoscopic ultrasound (EUS) could be one of the best methods with its applicability and reliability [2,3]. EUS has been used in cases where conventional CT or transabdominal ultrasonography (USG) fails and for the evaluation of intrahepatic lesions such as hepatocellular carcinoma (HCC) without typical radiological findings [4-7].
The aim of this study was to evaluate the role of EUS-guided fine needle aspiration (EUS-FNA) in the diagnosis of liver masses. Its impact on patient management and procedure-related complications were also reviewed.
MATERIALS AND METHODS
Between November 2017 and July 2018, patients who were referred for liver biopsy with EUS-FNA were included in the study. All patients were 18 years or older and diagnosed with a lesion in the liver by ultrasound, CT, or magnetic resonance imaging. All patients were informed about the procedure and informed consent was obtained. Ethical approval was obtained from the Institutional Review Board (No: 69/2019). Data of the patients were obtained from the hospital registry.
Patient selection
The selection criteria of the patients for EUS-FNA include the following: (1) patients with lesions suspected as HCC but not showing typical radiological appearances, (2) those with known extrahepatic malignancies (pancreas, colon, etc.) who were referred for confirmation of the metastases, (3) those who had masses in the gallbladder bed, and (4) those who had liver masses. EUS-FNA was planned as the first choice without any concern regarding the difficulty of the lesion access. Antiplatelet medications were discontinued at least 7 days before the procedure. The platelet count had to be above 50,000, and the international normalised ratio value had to be below 1.4 before the procedure. Informed consent was obtained from all patients.
Endoscopic ultrasound and endoscopic ultrasound-guided fine needle aspiration procedure
EUS-FNA was performed by a single endoscopist with a Fujinon echoendoscope (EG-580 UT; Fujinon, Tokyo, Japan). A 22-G needle was used for FNA (EZ Shot 2TM; Olympus Co., Ltd., Tokyo, Japan). Aspirations and biopsies were performed through the duodenum or stomach where the image and access to the lesion were the best. The needle was inserted into the lesion under EUS guidance (Fig. 1, Supplementary Videos 1, 2). After entering the lesion, the stylet was removed. The needle was moved back and forth within the lesion while applying negative pressure with a 10-mL syringe. The needle was moved forward and backward averagely 25–30 times within the lesion. Only a single pass was performed. Aspirated samples were evaluated by a single pathologist. On-site pathologist was not available during the procedures. Prophylactic antibiotherapy was not administered.
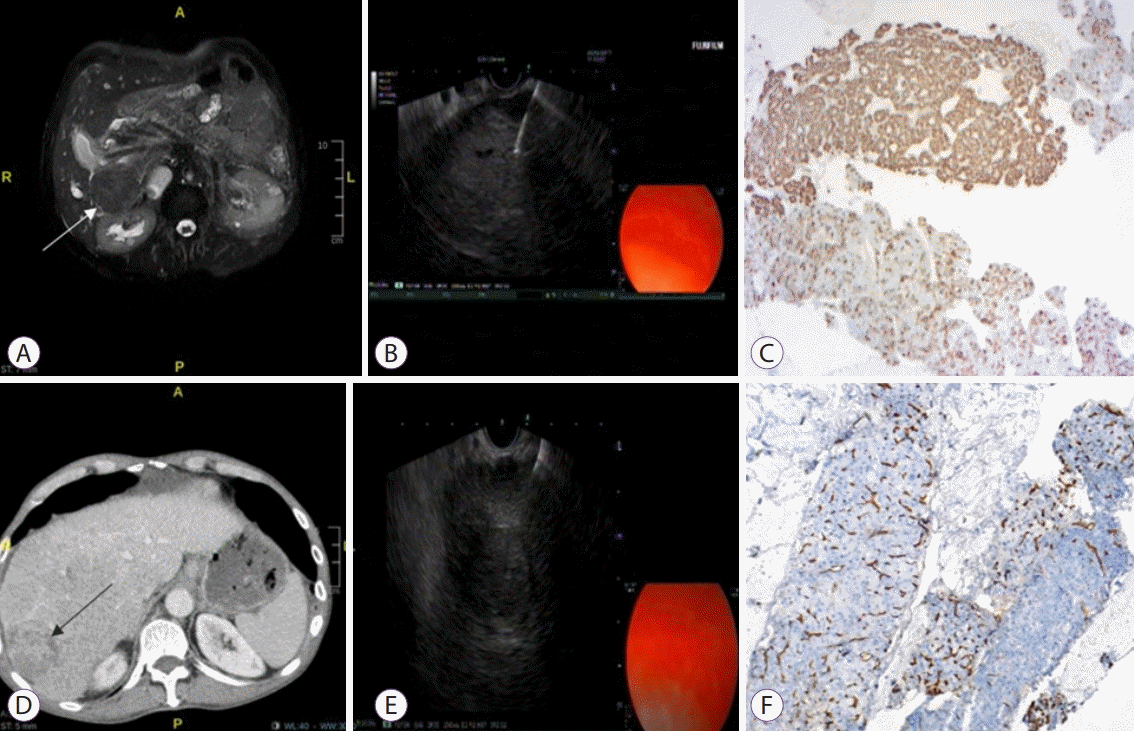
Performance of endoscopic ultrasound-guided biopsy from lesions in the right liver lobe. (A) A fat-suppressed T2-weighted magnetic resonance image shows an exophytic, isointense liver mass originating from segment 6. (B) An endoscopic ultrasound-guided fine needle aspiration (EUS-FNA) procedure to a 30 mm hyperechoic mass located in segment 6. (C) Diffusely positive staining is achieved in areas with acinar pattern, and focal staining is noted in thick trabecular areas with HepPar dye (×40). (D) Liver lesions in segment 6, showing intense heterogenous contrast enhancement at the early arterial phase on computed tomography. (E) EUS-FNA procedure to a 25 mm hyperechoic mass located in segment 6. (F) The hepatocellular carcinoma tissues located on the left side of the figure show an increase in capillarization unlike the normal parenchyma on the right side (CD34, ×40).
Cytological and histological evaluation
All patients had both air dried and alcohol-fixed preparations. Air dried preparations were stained with May–Grunwald–Giemsa stain and alcohol-fixed preparations were stained by Papanicolaou staining method. The tissue obtained for histological analysis was put in 10% buffered formalin (Fig. 2). Tissue particles taken together with the biopsy were centrifuged following fixation within the tissue solution. This concentrated and precipitated material was used for liquid-based cytology preparation, and the remaining material was converted into cell blocks.
Formalin-fixed tissues and cell blocks were stored as blocks after routine follow-up in the tissue tracking device. Hematoxylin and eosin (H&E) staining of sections was performed on the obtained tissues. During the preparation of the sections, for immunohistochemical (IHC)/histochemical tests, a minimum of 5 and a maximum of 10 synchronous sections were prepared on the lysine-coated plates. On average, 8 (range, 1–16) IHC and/or histochemical tests were performed on the obtained tissues.
Histochemical and immunohistochemical evaluation
Reticulin stain, pCEA, CD34, Glipican 3, and Ki67 IHC markers were utilized for the differential diagnosis of HCC. CK7, CK20, mCEA, HepPar 1, S100, and TTF1 were utilized for the differential diagnosis of adenocarcinoma. Immune markers like Chromogranin A, synaptophysin, and Ki67 were used for the differential diagnosis of neuroendocrine tumors (Figs. 3 and 4).

(A) A well-defined mass with an enhanced capsule in the liver, visualized at the venous phase of a contrast-enhanced, coronal fat-suppressed T1-weighted magnetic resonance image. (B) A 28×34 mm hypo-/iso-echoic mass in segment 6 during the endoscopic ultrasound-guided fine needle aspiration procedure. (C) Monotonous hepatocellular carcinoma cells stained with May–Grunwald–Giemsa (MGG) in the cytological specimen (MGG, ×400). (D) Tumor cells stained with hematoxylin and eosin (×200). (E) Increased capillarization and thick cellular trabeculae in the biopsy obtained under endoscopic ultrasound (CD34, ×200). (F) Alfafetoprotein (AFP) positive stained cells in the endoscopic ultrasound biopsy (AFP, ×200). (G) Macroscopic appearance of the resected tumor.
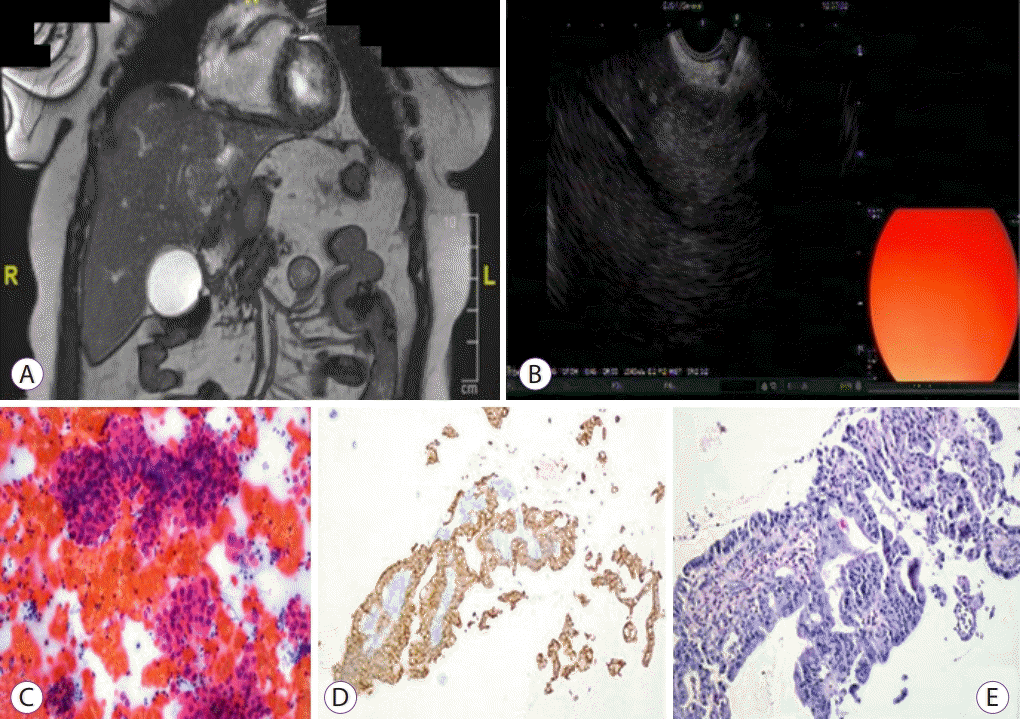
A cholangiocarcinoma evaluation. (A) An isointense hepatic mass in the left lobe having lobulated contours with infiltrative nature causing segmental intrahepatic bile duct dilatation in coronal T2-weighted 2D fast imaging employing steady-state acquisition (FIESTA) magnetic resonance image. (B) Endoscopic ultrasound-guided fine needle aspiration procedure to a 25 mm × 28 mm hyperechoic mass located in segment 4. (C) Tumor cells showing 3D groupings with Papanicolaou-stained aspiration (×200). (D) Tumor cells showing adenoid structures with CK7 positive staining in the biopsy material (×100). (E) Appearance of the tumor cells with hematoxylin and eosin stain (×200).
Following the first phase of staining, an additional one was also performed when required. For the IHC analysis, the XT ultraView DAB procedure was performed for staining in the Ventana BenchMark XT machine. Stained slides were closed with closing solutions after passing through alcohol (increasing grades) and xylene. Preparations were evaluated by a pathologist under a light microscope (Olympus BX53).
Classification of the results
Cytological and histological diagnoses were classified as follows: nondiagnostic, negative (benign), suspicious for malignancy, and malignancy positive. Cases suspicious for malignancy and malignancy-positive ones were classified as positive. Negative cases were classified as malignancy negative. Either histological or cytological positiveness was enough for consideration as malignancy positive.
In malignancy-positive cases, the definitive diagnoses were made according to surgical pathology results, USG-guided Tru-Cut biopsy results, and other clinicolaboratory findings. In benign lesions, the definitive diagnoses were made according to clinical and radiological findings during the 6th and 12th months of follow-up surveillance. According to these data, the diagnostic accuracy, sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of EUS-FNA were calculated. As reported previously, the impact of EUS-FNA was calculated considering all of the following: preventing unnecessary surgeries, role in the first diagnosis, upstaging the tumor, and modifying the patient management [1].
Statistical analysis
Categorical variables were described as frequency and n (%). Non-categorical (continuous) variables were described as mean ±standard deviation. All statistical analyses were calculated using the SPSS version 22.0 (SPSS Inc., Chicago, IL, USA) software.
RESULTS
A total of 25 patients who were referred to our center for the evaluation of liver lesions with EUS-FNA were included in the study. Demographics and clinical characteristics of the patients were presented in Table 1. The flowchart for patients included in the study was presented in Fig. 5. Six patients had chronic liver disease due to hepatitis B virus, and one had such disease due to hepatitis C virus. The median Alfa-fetoprotein level of these patients was 12.46 (7.20–42.4) ng/mL (IQR, 25–75). Three patients had ascites due to cirrhosis.

Characteristics of the Patients with Liver Mass Who Underwent Endoscopic Ultrasound-Guided Fine Needle Aspiration and Biopsy
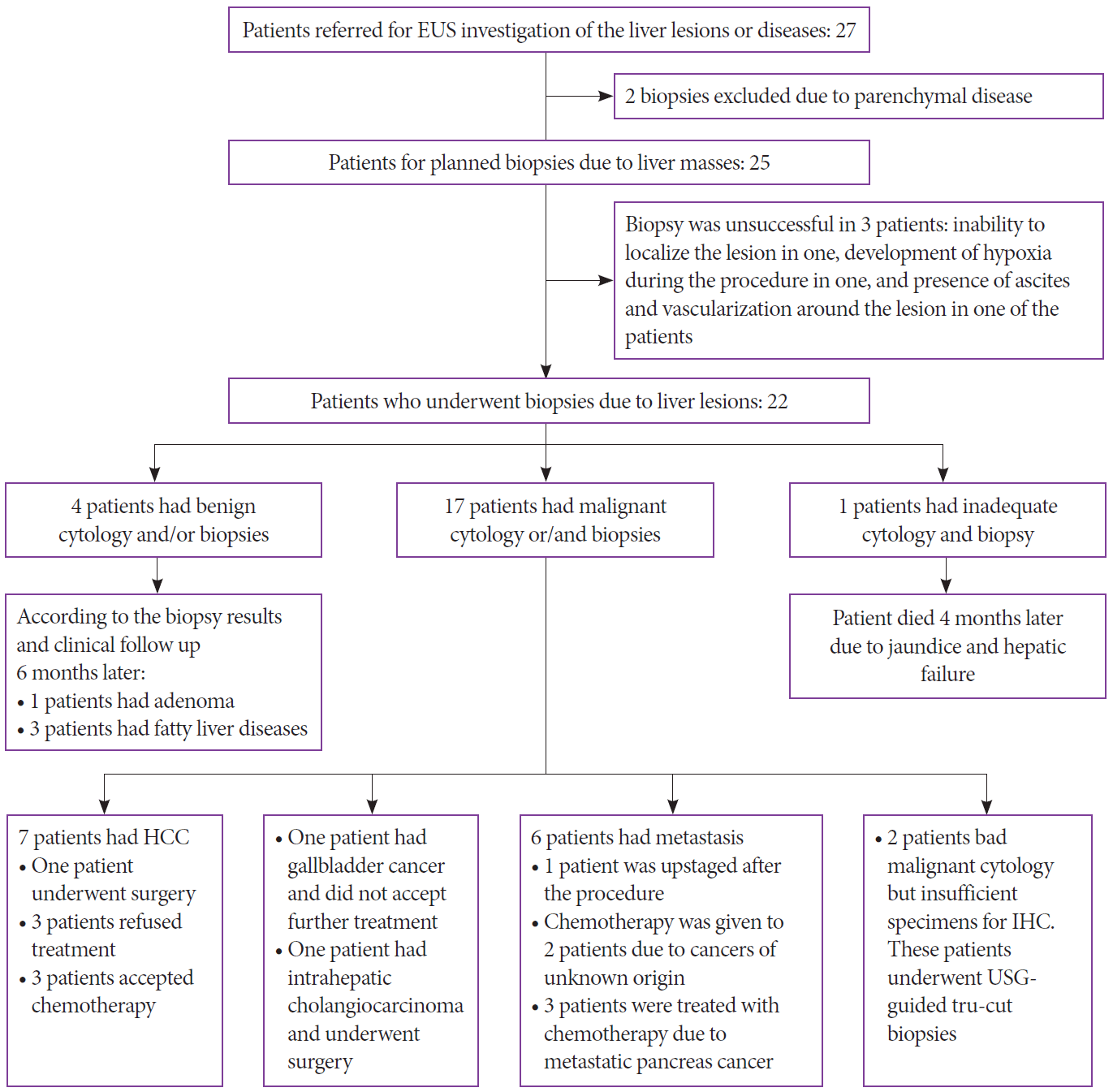
Flowchart of the patients with hepatic lesions. EUS, endoscopic ultrasound; HCC, hepatocellular carcinoma; IHC, immunohistochemical; USG, ultrasonography.
Biopsies were performed technically successfully in 22 (88%) patients. Of these, 11 (50%) were performed through the stomach, and the remaining 11 (50%) were performed through the duodenum. The lesion could not be localized in the right posterior lobe in one patient, the EUS procedure was stopped due to hypoxia in one patient, and biopsy was unsuccessful due to the vascularization around the lesion and presence of ascites in one patient. The features of the EUS evaluations are shown in Table 2.
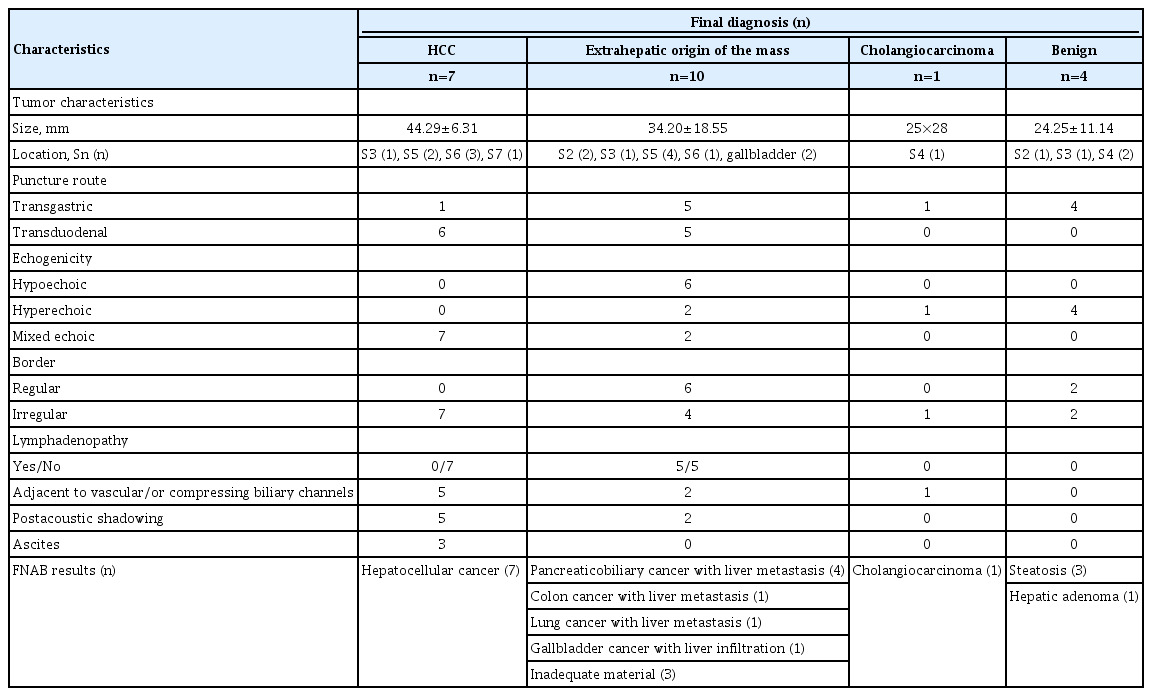
Endoscopic Ultrasound Features and Fine Needle Aspiration Biopsy Results according to the Final Diagnoses in 22 Patients Who Underwent Endoscopic Ultrasound-Guided Fine Needle Aspiration
The mean tumor size was calculated as 34.50±16.04 mm. Seven patients had more than one lesion, and 15 had single lesions. Of the 22 patients, 21 (95.45%) had sufficient aspirations, and 19 (86.3%) had sufficient biopsies for the H&E and IHC tests. Two (9%) patients had aspiration results consistent with malignancy; however, biopsies were insufficient for further analysis. These patients had the final diagnoses of linitis plastica and neuroendocrine tumor metastasis after USG-guided Tru-Cut biopsies. One (4.5%) patient had insufficient aspiration and biopsy. During the follow-up of this patient, the lesion in the gallbladder bed showed fast radiological progression leading to mechanical icterus and thus was diagnosed as gallbladder cancer. The patient was lost to follow-up at 4 months following the biopsy. The overall success rates of EUS-FNA and biopsies are listed in Table 3. The final diagnoses of hepatic lesions and methods of the diagnoses are summarized in Table 2.
Among patients with malignant cytology, EUS-FNA aspiration had clinical impact (according to definition) in 17 (100%) patients. EUS-FNA helped in the final diagnoses of 15 (83.3%) patients with malignant cytology. Out of 15 patients, 7 (46.7%) were diagnosed with HCC, one (6.7%) with gallbladder cancer, one (6.7%) with cholangiocarcinoma, and six (40%) with metastases. EUS-FNA helped in the diagnosis of liver metastasis in three (17.6%) patients, and these patients were referred for chemotherapy, thus avoiding surgery. Two (11.7%) patients were referred for surgery. Five (29.4%) patients (three HCC with ascites and two adenocarcinomas with unknown origin) were referred for chemotherapy due to inoperability.
Of the remaining five patients, four (three HCC and one gallbladder cancer) rejected the treatment, and one (lung adenocarcinoma) had upstaging. Overall, management was modified in 10 (59%) patients. Tru-Cut biopsy was needed in two patients (metastases) for the final diagnoses. EUS-FNA had sensitivity, specificity, NPV, and PPV rates of 94.4%, 100%, 80%, and 100%, respectively. The diagnostic accuracy for all lesions was calculated as 86.3% (19/22). There were no complications after the procedure such as bleeding or infection.
DISCUSSION
EUS-FNA has appeared as a key tool for the diagnostic evaluation, staging, and treatment of gastrointestinal tract lesions. In this study, we used EUS-FNA in liver lesions that were difficult to reach with conventional techniques and those with a high risk of complications. Through single pass, aspiration and biopsy sufficiencies were 95.4% and 86.3%, respectively. We did not encounter any complications. Overall, EUS-FNA modified the management of 10 (59%) patients.
Both primary liver lesions and liver metastases may have important impacts on the management and prognosis of the patients. CT is a standard imaging modality in the detection of liver masses. EUS is a well-described test for the diagnosis and staging of gastrointestinal and lung tumors and has become an alternative tool for liver imaging [8]. Nguyen et al. were the first to report that CT was able to demonstrate only 21% of the liver lesions detected by EUS [9]. They showed that EUS could detect liver metastases that could not be detected by CT and this could change the M stage of a disease and its management [9]. Awad et al. reported that EUS investigation and FNA from the liver lesions changed the management in 67% of the patients [4]. Dewitt et al. reported 86% change after EUS-FNA in the management of solid liver lesions in 77 patients [1]. In our series, this ratio was 59%. Rejection of the treatment by four of our patients may have caused the decreased rate. When four patients who rejected the treatment were added to the calculation, the impact on the patient management rate increased to 82%.
Whether ultrasonographic features of the lesions that are evaluated by EUS could predict if they are benign or malignant is of utmost importance. tenBerge et al. reported that the shape, size, echogenicity, and features of the borders of the lesions were not predictable in the differentiation between benign and malignant lesions [10]. Fujii-Lau et al. developed a scoring system according to the endosonographic features with 88% PPV rate and reported that this could help in deciding on which lesions should be biopsied or not [11]. In this series, we saw that geographically shaped lesions were benign and malignant lesions had more surrounding vascular and biliary channel distortions and greater post-acoustic shadowing. Moreover, the echogenicity and border features of the lesions were not helpful in the differentiation between benign and malignant lesions (Table 2). Therefore, we suggest that once a lesion is demonstrated, FNA should be performed regardless of its echogenicity.
The diagnostic accuracy of EUS-FNA in hepatic masses was reported between 82% and 100% [12]. Lee and colleagues reported 90.5% and 86.3% diagnostic yields after core needle biopsies for liver lesions, in malignant and specific tumors, respectively [13]. In our study, the diagnostic yield rate for all lesions was calculated as 86.3%. In a prospective study carried out by Hollerbach et al., the sensitivity, specificity, NPV, and PPV rates were 94%, 100%, 78%, and 100%, respectively [14]. In this study, these rates were 94.4%, 100%, 80%, and 100%, respectively.
In our study, with single pass, we obtained sufficient aspirates in 95.4% and sufficient biopsies for necessary stainings in 86.3% of the patients. Nguyen et al. reported a mean of two passes and diagnostic accuracy of 100% [9]. Moreover, Oh et al. reported a mean pass number of 3, technical success rate of 97.9%, and sufficient aspirate rate of 91.3% [15]. To achieve an acceptable success rate with one pass, we performed 25–30 times to-and-fro movements within the lesion. During the procedure, we did not have an on-site cytopathologist. It could be proposed that reduced pass numbers may shorten the procedure duration, decrease complications like bleeding, and reduce seeding risk.
The most challenging parts of the liver to visualize during EUS are the right posterior and anterosuperior segments [10]. The gallbladder and portal vascular structures interfere with the vision during the procedure in these regions. Oh et al. compared the right and left lobe EUS-FNA procedures and reported diagnostic yield rates of 89.3% and 92.9%, respectively [15]. In one patient, the lesion was not able to localize in the right posterior lobe. In our series, the lesion was not visualized in one patient due to its localization in the posterior part of the right lobe. By increasing the tissue depth with low frequencies (5 Mhz), the problems with transbulbar and transduodenal approach could be overcome. Despite this limitation, EUS-FNA has some advantages over the percutaneous USG-guided needle biopsy technique, including less influence from the respiration, performance in less cooperative patients, performance in obese patients and those with massive ascites, and ease in approaching the caudate lobe. In addition, compared with USG-guided percutaneous biopsies, less post-procedural pain was reported in EUS-guided liver biopsies [16].
As the experience with EUS-guided liver biopsy increases, newly designed needles for this procedure emerge. Eskandari et al. compared needles with different geometric tips and sizes of 19-, 20-, 22-G in the fine needle aspiration biopsy (FNAB) procedure in a freshly harvested bovine liver and showed that 19-G needles had higher diagnostic performance compared with 20-G needles [17]. In a randomized study with cadaveric livers, Schulman et al. reported that biopsies with 22-G needles had higher numbers of portal tracts compared with those with 19-G needles [18]. Because either of the studies was not performed in real patients, it is not possible to obtain any information about the adverse events. In this study, with the help of EZ Shot 2 22-G needle, we succeeded to achieve a higher amount of FNAB material. The presence of side hole in its specifically designed tip may have helped in our high yield.
The major complication rates with 22- or 24-G needles were reported to be between 0% and 4% [12]. Major complications include infection, hemorrhage, biliary peritonitis, and rarely malignant cell seeding [19]. In a retrospective series with 167 patients, the major complication rate of EUS-FNA in hepatic lesions was reported to be approximately 1%. In this series, there were two patients with abdominal pain and two with fever, and one patient died [10]. In a meta-analysis, it was reported that the EUS-FNA procedure had a greater risk in the liver than in other localizations, and this risk increased with ascites [20]. In this series, we did not encounter any complications. We had three cirrhotic patients with ascites, and their platelet count was between 50,000 and 70,000. These results were in favor of the safety of the EUS-FNA procedure for liver biopsy.
The main limitations of this study were its retrospective design and limited sample size. However, the presence of the same specialists during both EUS procedure and pathological investigations could be its strengths. Increased successful biopsy rates accompanying the increased successful cytology rates further enriched our results. Several types of liver lesions, both benign and malignant, were included in this study.
In summary, EUS-FNA for liver lesions have high success rates in tissue sample retrieval and lower complication rates even in patients with ascites. This procedure should be considered for liver lesions that are hard to reach with the conventional methods or bearing high complication risks.
Notes
Conflicts of Interest: The authors have no potential conflicts of interest.
Funding
None.
Supplementary Material
Video 1. Endoscopic ultrasound-guided fine needle aspiration procedure from the lesion in the right lobe (https://doi.org/10.5946/ce.2020.065.v001).
Video 2. Endoscopic ultrasound-guided fine needle aspiration procedure from the lesion in the left lobe (https://doi.org/10.5946/ce.2020.065.v002).


