Diode Laser—Can It Replace the Electrical Current Used in Endoscopic Submucosal Dissection?
Article information
Abstract
Background/Aims
A new medical fiber-guided diode laser system (FDLS) is expected to offer high-precision cutting with simultaneous hemostasis. Thus, this study aimed to evaluate the feasibility of using the 1,940-nm FDLS to perform endoscopic submucosal dissection (ESD) in the gastrointestinal tract of an animal model.
Methods
In this prospective animal pilot study, gastric and colorectal ESD using the FDLS was performed in ex vivo and in vivo porcine models. The completeness of en bloc resection, the procedure time, intraprocedural bleeding, histological injuries to the muscularis propria (MP) layer, and perforation were assessed.
Results
The en bloc resection and perforation rates in the ex vivo study were 100% (10/10) and 10% (1/10), respectively; those in the in vivo study were 100% (4/4) and 0% for gastric ESD and 100% (4/4) and 25% (1/4) for rectal ESD, respectively. Deep MP layer injuries tended to occur more frequently in the rectal than in the gastric ESD cases, and no intraprocedural bleeding occurred in either group.
Conclusions
The 1,940-nm FDLS was capable of yielding high en bloc resection rates without intraprocedural bleeding during gastric and colorectal ESD in animal models.
INTRODUCTION
Endoscopic submucosal dissection (ESD) is an advanced endoscopic technique used to remove superficial gastrointestinal (GI) tract neoplasms. This technique has advantages enabling en bloc resection regardless of the tumor size [1]. The resection margins of tissue removed en bloc can be accurately evaluated in all directions by pathologists. Thus, local recurrence can be reduced by determining whether the lesions are completely resected [2]. Despite these advantages, ESD is not popular worldwide because of technical difficulties and high-risk complications. Thus, various types of endoscopic knives have been developed to overcome these technical difficulties; these include insulated tip knife-type (IT knife, IT knife 2, and IT knife nano), needle knife-type (hook knife, dual knife, flex knife, B-knife, and hybrid knife), and grasping scissor knifetype (SB knife, SB knife Jr, and Clutch cutter knife) [1,3]. These endoscopic knives have helped remove lesions more safely and effectively but can cause intraprocedural or postprocedural bleeding because of the electrosurgical current.
Bleeding is one of the most common complications associated with ESD, as intraprocedural bleeding interferes with precise endoscopic resection under a clear view of the operating field, which can lead to longer procedure times or an increase in the frequency of complications, such as perforation [4]. The incidence rates of immediate and delayed bleeding associated with gastric ESD are approximately 22% and 4.5%–5.5%, respectively [5-7]. A recent meta-analysis showed that the incidence rates of immediate and delayed bleeding associated with colorectal ESD are 0.82% and 1.7%, respectively [8].
Lasers generate heat and cause edema in the vessel wall, resulting in hemostasis [9]. Therefore, attempts have been made to reduce the risk of bleeding associated with these laser properties by using several types of lasers, including carbon dioxide (CO2) laser [10,11], Nd: YAG laser [12], thulium laser [13], and diode laser [14], for endoscopic procedures.
The diode laser system has been used as a surgical instrument for incising and dissecting tissue and for achieving hemostasis. However, no study has yet performed ESD using the 1,940-nm diode laser system. A new medical fiber-guided diode laser system (FDLS) designed for endoscopic procedures emitting a high-frequency 1,940-nm wavelength is expected to offer high-precision cutting with simultaneous hemostasis. Thus, this study aimed to evaluate the feasibility of the application of the FDLS for performing ESD in the GI tract of an animal model.
MATERIALS AND METHODS
This study was designed as a prospective animal pilot study. Herein, the feasibility of endoscopic application of the FDLS in ESD was evaluated in ex vivo and in vivo porcine models. An ex vivo porcine model was used first to confirm the feasibility of FDLS application for ESD, followed by an in vivo porcine model to assess its clinical applicability.
Instruments
A single-channel endoscope (GIF-Q260J; Olympus Medical Systems Co., Tokyo, Japan) was used for all ESD procedures. A transparent distal cap (Olympus EndoTherapy; Olympus America Inc., Waltham, MA, USA) was attached to the tip of the endoscope to optimize visualization. A hypertonic saline solution mixed with diluted epinephrine and traces of indigo carmine were injected to lift the mucosal and submucosal layers.
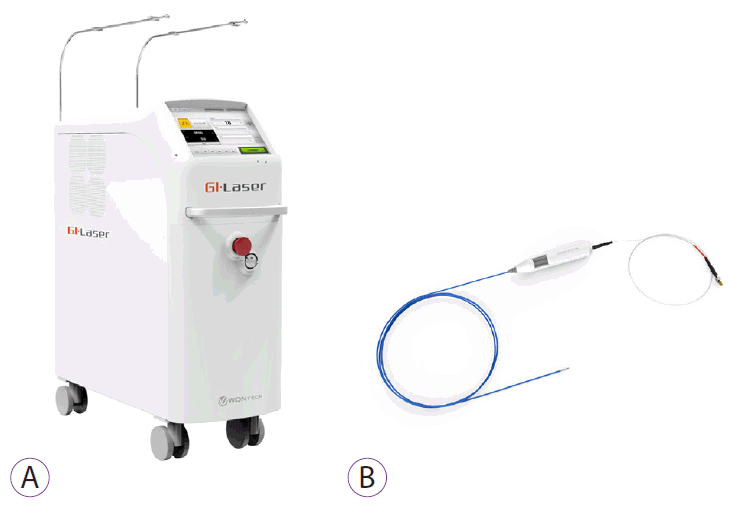
A laser system (GI Laser; Wontech, Daejeon, Korea) emitting a high-frequency diode laser (1,000 Hz) and 1,940-nm wavelength was used for ESD. A specially designed fiber optic laser catheter (GI Laser Knife; Wontech) was used to protect and deliver the laser fiber to the surgical location through the working channel of the endoscope. The length of the fiber tip of this device can be adjusted by the operator according to the type of procedure, and up to 18 W of output can be delivered to the target tissue (Fig. 1).
Colonic endoscopic submucosal dissection using the fiber-guided diode laser system in the ex vivo porcine model
This experiment was exempt from institutional review board approval because no human research participants or live animals were involved. Fresh ex vivo porcine colorectal specimens, which included the rectum, sigmoid, and descending colon, were obtained from a commercial distributor. The length of the colon specimens was 40 cm, and their diameter ranged from 6 to 8 cm. The specimens were used in the EASIE-R simulator platform (EndoSim, LLC, Berlin, MA, USA).
Ten standardized artificial lesions measuring 3×3 cm were created using an endoscopic ruler approximately 15 cm from the anal verge in the porcine colorectal specimens. Thereafter, colonic ESD was performed using the FDLS by an experienced endoscopist who had performed more than 500 cases of human gastric or colorectal ESD (Supplementary Video 1).
Gastric and rectal endoscopic submucosal dissection using the fiber-guided diode laser system in the in vivo porcine model
Two mini pigs (Sus scrofa; Medikinetics Co., Ltd., Pyeongtaek, Korea; mean body weight, 30 kg; mean age, 14 months) were prepared for the in vivo experiment. All animal experiments were performed at the NCEED large animal endoscopy center after the experimental protocol was approved by the Institutional Animal Care Committee of KNOTUS Co., Ltd., a contract research organization for preclinical testing, before commencement of the study (KNOTUS IACUC 19-KE-359). Before testing, each animal was anesthetized with an intramuscular injection of tiletamine/zolazepam and xylazine. Eight ESD procedures (four gastric and four rectal ESD procedures) were performed by eight endoscopists who had performed more than 300 cases of human gastric or colorectal ESD (Supplementary Video 2).
Endoscopic submucosal dissection procedures
The procedures were performed using the FDLS and the following steps instead of conventional endoscopic knives: artificial lesions 3 cm in diameter were created at sites in the stomach or rectum easily reached by the endoscope by marking using the FDLS. A 0.9% normal saline solution combined with 0.05% indigo carmine was injected to the submucosal space at the target lesion. In the gastric ESD cases, a circumferential incision was created a few millimeters outside the margins of the target lesion. Thereafter, submucosal dissection was performed until the lesion was completely removed. In the colorectal ESD cases, a mucosal injection to the anal side of the tumor and a partial mucosal incision were performed. Submucosal dissection was then performed to prepare a mucosal flap. After the creation of the flap, half to two-thirds of the dissection was performed, followed by a full circumferential mucosal incision. Thereafter, submucosal dissection was performed until the lesion was completely removed.
Main outcome measurements
After we retrieved the excised lesion, the specimen and resection bed were inspected to determine the en bloc resection status. The specimens were spread and pinned on flat cork plates immediately after resection. The largest and perpendicular diameters of the specimen and resection area were measured. The procedure time, injection volume, and injection frequency were recorded, and the incidence of adverse events (bleeding and perforation) was monitored by an independent observer. The procedure speed, defined as the area of mucosa dissected per unit time (cm2/min), was calculated. All pigs were sacrificed after the procedures using excess isoflurane, and the stomach and colon of each pig were harvested for inspection and histopathological examination. These procedures were performed by a pathologist who was blinded to the study groups.
Perforations were assessed by searching for visible holes in endoscopic view or the harvested organs, using the underwater test, and by evaluating histopathologically. The degree of injury to the muscularis propria (MP) layer was histologically classified into four categories: grade 1 (mild), involving <50% of the inner circular layer; grade 2, involving >50% of the inner circular layer but not reaching the outer longitudinal layer; grade 3, involving <50% of the outer longitudinal layer; and grade 4, involving >50% of the outer longitudinal layer and considered a histopathological perforation. The degree of injury to the MP layer per remaining tissue bed was recorded [14].
Statistical analysis
Data were presented as means±standard deviations and were evaluated for differences using the SPSS software, version 18.0 (SPSS Inc., Chicago, IL, USA). The independent samples t-test was used to compare the results between the gastric and colonic ESD groups. Categorical variables were compared using Fisher’s exact test. A p-value of <0.05 was considered significant.
RESULTS
Ex vivo animal experiments
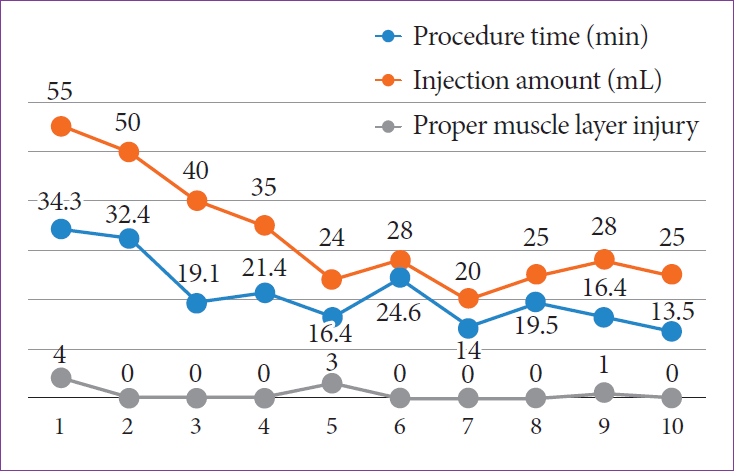
All 10 colonic ESD procedures were completed using the FDLS without an endoscopic knife. The mean size of the resected specimens was 4.1×4.0 cm. The mean total procedure time and speed were 21.2±7.3 (range, 13.5–34.3) min and 0.87±0.31 (range, 0.38–1.31) cm2/min, respectively. The mean injection volume and number of MP layer injuries were 33.0±11.8 mL and 0.8±1.5, respectively. The en bloc and complete resection rates were both 100% (10/10), and perforation occurred in the first case only (Table 1). The mean injection volume was significantly larger (p=0.046) in the first five cases than in the last five cases. The mean procedure time (25.2±8.1 min vs. 40.8±12.2 min) and number of MP layer injuries (0.2±0.4 vs. 1.4±1.9) tended to be shorter and lesser, respectively, in the last five cases than in the first five cases (p>0.05) (Figs. 2 and 3).
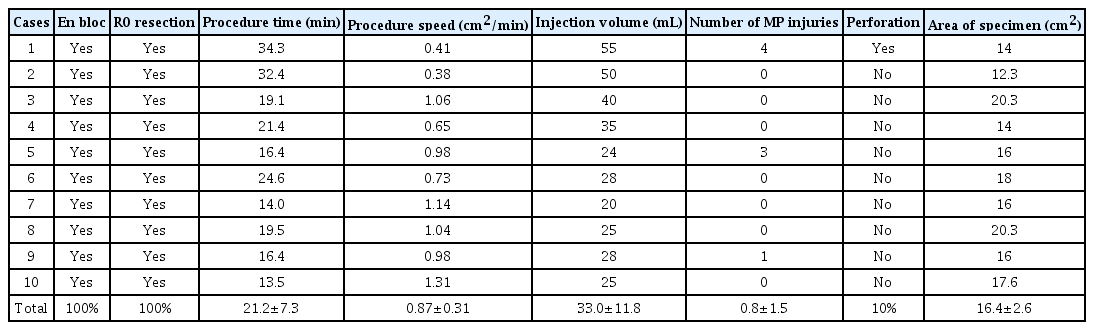
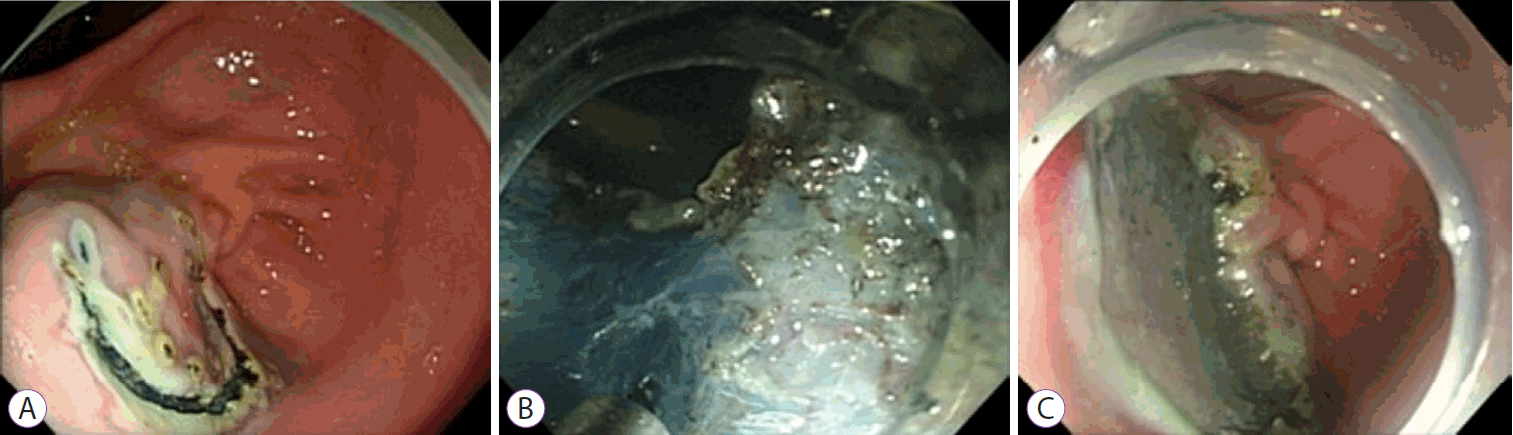
Results of Colonic Endoscopic Submucosal Dissection Using a Fiber-Guided Diode Laser System in the ex vivo Model
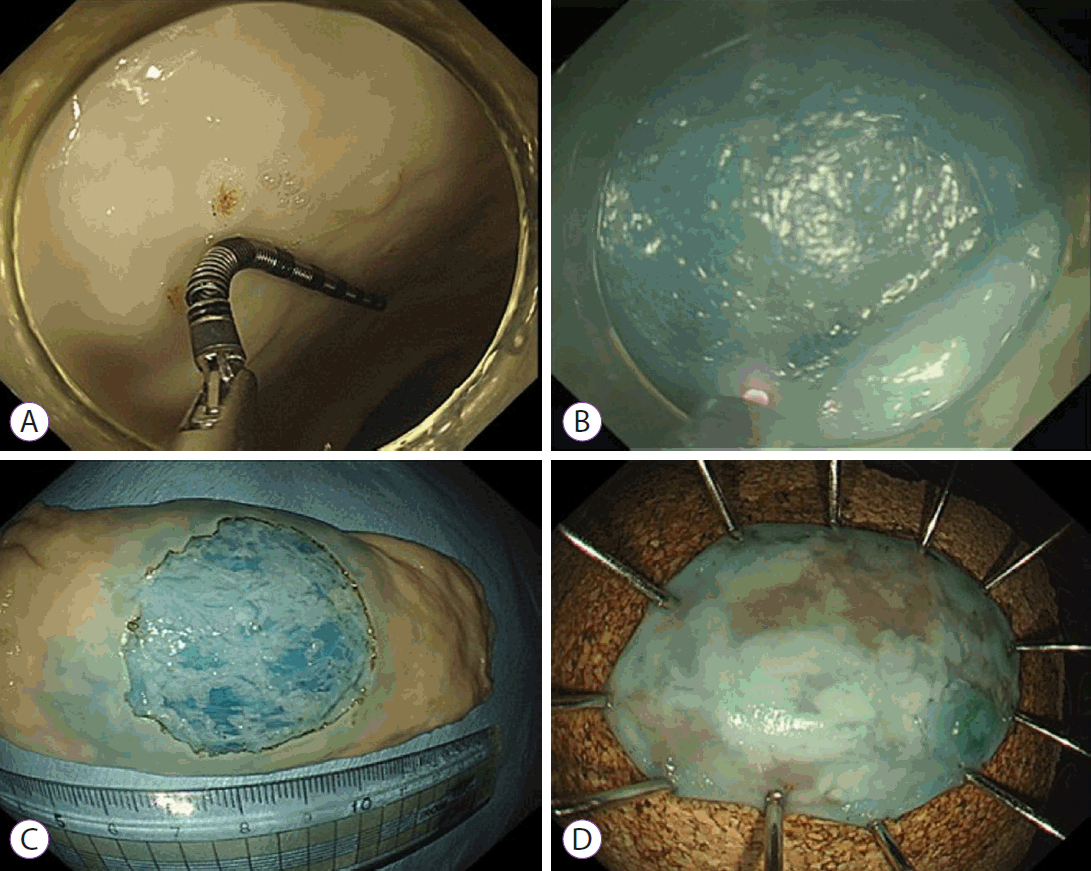
Endoscopic submucosal dissection using the fiber-guided diode laser system in the ex vivo model. (A) Creation of artificial lesion 3 cm in diameter using an endoscopic ruler. (B) Submucosal dissection using the fiber optic laser catheter. (C) Remaining porcine colon tissue bed after endoscopic resection. (D) Specimen spread and pinned on cork plates immediately after resection.
In vivo animal experiments
Eight ESD procedures (four gastric ESD and four rectal ESD procedures) were completed via en bloc resection using the FDLS without an endoscopic knife. The mean marginal cutting time and submucosal dissection time were 4.5±5.1 min and 21.0±4.4 min, respectively, in the gastric ESD group. The mean total procedure time, procedure speed, and specimen area were 27.7±7.3 min, 0.42±0.10 cm2/min, and 11.1±1.3 cm2 in the gastric ESD group and 28.5±5.8 min, 0.42±0.09 cm2/min, and 11.6±1.2 cm2 in the rectal ESD group (Tables 2 and 3).
Results of Gastric and Rectal Endoscopic Submucosal Dissection Using a Fiber-Guided Diode Laser System in the in vivo Model
In the gastric ESD group, no MP layer injury occurred in 50% (2/4) of the cases, and grade 1 MP layer injury occurred in 50% (2/4) of the cases. In the rectal ESD group, MP layer injury did not occur in 25% (1/4) of the cases; however, grade 1, 2, and 4 MP layer injuries occurred in 25% (1/4) of the cases each. One case of perforation occurred only in the rectal ESD group. No intraprocedural bleeding occurred in either group (Fig. 4).
DISCUSSION
ESD is one of the most technically difficult endoscopic procedures. According to the recently updated guidelines of the Japan Gastroenterological Endoscopy Society, understanding anatomical features and familiarity with the basic techniques of polypectomy, endoscopic mucosal resection, endoscopic hemostasis, and clip suture are the minimum requirements before starting ESD [15]. Novices must practice using ex vivo or in vivo animal models or in the clinic under a supervisor to perform ESD more safely and efficiently. Several animal and clinical trials reported that at least 30 cases of gastric ESD should be performed to achieve an acceptable technical level [16,17]. However, one study reported that a high technical level cannot be reached until more than 240 cases of colorectal ESD have been performed [18].
Several factors increase the difficulty of ESD, including intraprocedural bleeding, paradoxical scope control, non-lifting lesions, perpendicular submucosal approach, ineffective gravity countertraction, and submucosal fibrosis [19]. The incidence of intraprocedural bleeding during ESD is >20% [20], and proper management of bleeding plays a critical role in successful ESD because it interferes with precise cutting under clear vision.
Several studies have evaluated the clinical effectiveness and applicability of various types of laser devices capable of precise tissue cutting without intraprocedural bleeding during ESD. Cho et al. [13] reported preliminary results of gastric ESD using thulium laser. Ten patients underwent ESD using thulium laser without an endoscopic knife, and the curative resection rate was 90% (9/10). No active intraprocedural bleeding, delayed bleeding, or perforation was detected. Preliminary results of gastric ESD were also reported with the use of CO2 laser in ex vivo and in vivo porcine models. En bloc resection was achieved in all eight gastric ESD (five ex vivo and three in vivo) cases, and there was no perforation, MP layer injury, or uncontrollable hemorrhage observed during the procedures [11].
The diode laser system has been used for a variety of surgeries, such as prostate disease surgery [21], endovenous ablation treatment [22], tonsillectomy [23], and laser-assisted laparoscopic partial nephrectomy [24], and has proven to be effective for tissue excision and hemostasis. Tang et al. [14] demonstrated the feasibility of ESD using diode laser in an animal model. Eight cases of esophageal ESD were performed using the synchronous 980/1,470-nm dual-diode laser system, achieving a 100% en bloc resection rate without perforation. The procedure speed of ESD was significantly faster using the dual-diode laser system than using a conventional endoscopic knife (0.27 cm2/min vs. 0.21 cm2/min, p=0.001). In addition, fewer intraprocedural bleeding points were detected, and the use of hemostatic forceps was significantly less frequent in the diode laser ESD group than in the conventional ESD group. However, studies on diode laser-assisted ESD are very rare. The 1,940-nm diode laser system, as demonstrated in tonsillectomy, is capable of precise excision of the mucosa and submucosa without intraoperative bleeding; however, no studies related to endoscopic procedures have been published [23]. To apply these advantages of diode laser to ESD, we conducted gastric or colorectal ESD using a specially designed fiber optic laser catheter that enables tissue cutting through the endoscope working channel, similar to an endoscopic knife.
In our ex vivo study, en bloc and complete resections were achieved in all 10 cases of colonic ESD using the FDLS. Perforation occurred in the first case of colonic ESD because it was initially difficult to maintain the proper cutting direction using the FDLS. Afterwards, only minor MP layer injuries occurred, without any perforations in the remaining cases. As the operator adapted relatively quickly, the number of MP layer injuries (0.2±0.4 vs. 1.4±1.9) and procedure time (25.2±8.1 min vs. 40.8±12.2 min) were lower and shorter, respectively, and the procedure speed (1.04±0.21 cm2/min vs. 0.70±0.31 cm2/min) was faster in the last five cases than in the first five cases.
In our in vivo study, the en bloc resection and perforation rates were 100% and 12.5%, respectively, although most endoscopists had no experience with ESD using the FDLS. No intraprocedural bleeding occurred; however, MP layer injuries were more frequent during rectal ESD (25% grade 1, 25% grade 2, and 25% grade 4 injuries) than during gastric ESD (50% grade 1 injury). These results probably occurred because the colon wall is thinner than the stomach wall, and the colon lumen is narrower than the stomach lumen, making endoscopic manipulation difficult.
Based on the results of our study, FDLS-guided ESD has some advantages, such as a low risk of bleeding and safe cutting in the area of submucosal injection. Intraprocedural bleeding did not occur in this study. If it had occurred, it is expected that hemostasis would have been achieved with the FDLS using the non-contact technique without changing to other hemostasis devices. The FDLS enabled cutting the mucosa or submucosa very safely after the submucosal injection because the output wavelength of a 1,940-nm laser coincides with the absorption peak of water. In addition, the high absorption coefficient of a 1,940-nm laser allows a shallow penetration depth, enabling precise ablation with less thermal damage [23,25]. When the resected specimens were evaluated histologically, the cutting surface was observed accurately enough to judge whether the lesion was completely excised (Fig. 5).

Histopathological examination in the in vivo study. (A) Resected colon tissue specimen (hematoxylin and eosin staining, ×12.5 magnification), (B) remainder of the stomach after endoscopic resection (hematoxylin and eosin staining, ×12.5 magnification).
However, there were some disadvantages when using this device. The FDLS-guided ESD technique differs from the conventional ESD technique. Conventional needle-type ESD knives enable tissue resection of any tissue in contact with the metal tip; however, the apex of the tip can be used to resect the tissue when using the fiber-optic laser catheter. This limitation made it technically difficult to secure an appropriate resection plane when the lesion was in a perpendicular position. Thus, it was important to maintain a safe resection plane parallel to the MP layer during submucosal dissection.
Some limitations of this study should be discussed. The sample size was small, and we did not reflect the condition of the patients’ breathing or heartbeat. We selected the optimal location for the ESD procedure and did not compare it with the use of conventional endoscopic knives. Despite these limitations, this is the first study to investigate the usefulness of the 1,940-nm FDLS for gastric and colorectal ESD. Large animal trials or human clinical trials are needed to confirm the advantages of this device over conventional endoscopic knives.
In conclusion, the 1,940-nm FDLS was capable of yielding a high en bloc resection rate without intraprocedural bleeding during gastric and colorectal ESD in an animal model.
Notes
Conflicts of Interest: Wontech Co., Ltd. (Daejeon, Korea) provided the FDLS and fiber optic laser catheter. The authors have no potential conflicts of interest.
Funding
None.
Author Contributions
Conceptualization: Yunho Jung, Gwang Ho Baik, Weon Jin Ko, Joo Young Cho
Data curation: YJ, GHB, WJK, Bong Min Ko, Seong Hwan Kim, Jin Seok Jang, Jae-Young Jang, Wan-Sik Lee, Young Kwan Cho, Sun Gyo Lim, Hee Seok Moon, In Kyung Yoo, JYC
Formal analysis: YJ
Investigation: BMK, SHK, JSJ, JYJ, WSL, YKC, SGL, HSM, IKY
Methodology: JYC
Project administration: GHB, JYC
Resources: WJK, JYC
Writing-original draft: YJ, JYC
Writing-review&editing: GHB
Supplementary Material
Video 1. Ex vivo colon endoscopic submucosal dissection using fiber-guided diode laser system (https://doi.org/10.5946/ce.2020.229.v001).
Video 2. In vivo gastric endoscopic submucosal dissection using fiber-guided diode laser system (https://doi.org/10.5946/ce.2020.229.v002).