Incidence of Infection among Subjects with Helicobacter pylori Seroconversion
Article information
Abstract
Background/Aims:
Helicobacter pylori (H. pylori) seroconversion may occur during screening for gastric cancer. Our study aimed to assess the number of seroconverted subjects with H. pylori and their results in follow-up tests.
Methods:
Data were consecutively collected on subjects who were H. pylori-seronegative and presented for gastric cancer screening. Subjects who were followed up using the same serology test and pepsinogen (PG) assays on the day of endoscopy were included in the study.
Results:
During the follow-up of 57.7±21.4 months, 61 (15.0%) of 407 seronegative subjects showed seroconversion. H. pylori infection was detected in six (9.8%) of 61 seroconverted subjects. A diffuse red fundal appearance, with a significant increase in the Kyoto classification scores for gastritis, was observed in the infected subjects (p<0.001). Compared to the false-seropositive subjects, infected subjects showed higher serology titers (p<0.001) and PG II levels (p<0.001) and lower PG I/II ratios (p=0.002), in the follow-up tests.
Conclusions:
Seroconversion occurred in 3.3% of seronegative subjects per year; however, only 9.8% had H. pylori infection. The majority (90.2%) of the seroconverted subjects showed false seropositivity without significant changes in the follow-up test results. The diffuse red fundal appearance could be an indicator of H. pylori infection.
INTRODUCTION
Serum assays are often combined with gastroscopic examination in gastric cancer screening. Gastric biopsy allows for accurate detection of the presence of Helicobacter pylori; however, false-negative findings are not rare [1]. Indeed, the risk of false-negative Giemsa staining results is increased in patients with decreased gastric secretory ability [2]. Therefore, in addition to gastroscopy, serum anti-H. pylori immunoglobulin G (IgG) titers are often monitored, along with serum pepsinogen (PG) levels [3]. Although the specificity of the serology test was lower, the sensitivity was higher than in other H. pylori tests [4].
Gastroscopy findings and serum assay results help predict the status of H. pylori infection [5]. However, in an H. pylori-seroprevalent population, a considerable proportion (22.2%) of seronegative subjects with normal PG assay findings had past infection [6]. Moreover, seronegative subjects often demonstrate closed-type (22.8%) or open-type (6.0%) atrophic gastritis, even in the absence of gastric corpus atrophy (serum PG I ≤70 ng/mL and PGI/II ≤3) [7]. A diffuse red fundal appearance, mucosal swelling, nontransparent sticky mucus, and hypertrophic gastric rugae in the corpus are well-known findings of active H. pylori infection; alternatively, regular arrangement of collecting venules (RAC), linear streaks, and fundic gland polyps are more common in the absence of H. pylori infection [8].
It is important to collect data of false-seropositive subjects without infection and those infected with H. pylori during the follow-up of seronegative individuals. A recent study indicated that the annual seroconversion rate was 2.79%, and that living with an H. pylori-infected individual was a risk factor for seroconversion in Koreans [9]. It is important to accurately identify H. pylori infection among seroconverted subjects; however, data during screening for gastric cancer are limited in Korea. In this study, we aimed to accurately determine the number of subjects accurately diagnosed with H. pylori after seroconversion in follow-up tests. Furthermore, we aimed to identify the differences between H. pylori-infected subjects and those with false-seropositive results.
SUBJECTS AND METHODS
Selection of study subjects
After our previous study, we followed-up a consecutive series of Koreans subjects until 2019 who were H. pylori-seronegative and underwent screening for gastric cancer at our center between 2010 and 2014 [10]. Among the subjects registered at ClinicalTrials.gov (NCT01824953), only those who were followed up using the same H. pylori serologic test for ≥24 months were analyzed in this study. Subjects with a recent history of H. pylori eradication within the past two years, renal failure, or a history of gastrectomy were excluded from the study.
This study was performed at our center in accordance with the Helsinki Declaration after Institutional Review Board approval (KUH1010626). Informed consent was obtained from the subjects before performing gastric cancer screening tests using GIF-H260, GIF-H290 (Olympus, Tokyo, Japan), or EG-2990i (Pentax, Tokyo, Japan) endoscopes; serum PG assay (HBi Co., Anyang, Korea); and serum anti-H. pylori IgG Chorus assay (DIESSE Diagnostica Senese, Siena, Italy). All digital data were collected using ethical methods.
Assessment for endoscopy and gastric biopsy findings
Based on the Kyoto classification scoring system for gastritis, the presence and degree of chronic atrophic gastritis (A), metaplastic gastritis (IM), hypertrophic gastric rugae (H), nodular gastritis (N), and diffuse red (DR) fundal appearance were scored from 0 to 8 [11]. Briefly, chronic atrophic gastritis was scored as A0 (none or closed-type 1), A1 (closed-type 2 or 3), and A2 (open-type). Metaplastic gastritis was scored as IM0 (none), IM1 (limited), or IM2 (extensive). Hypertrophic rugae were scored as H0 (absent) and H1 (present). Nodular gastritis was scored as N0 (absent) or N1 (present). The diffuse red fundal appearance was scored as DR0 (none), DR1 (mild), and DR2 (severe).
Endoscopic biopsies were performed on lesions with abnormal shapes or discoloration. Gastric biopsy specimens were subjected to hematoxylin and eosin staining, as well as Giemsa staining. H. pylori-related gastric biopsy findings have been reported as previously described [12]. H. pylori infection was confirmed based on positive Giemsa staining.
Determining H. pylori infection status
Infection status was classified as H. pylori-naïve, past, or current. Past infection was defined as either a history of eradication, H. pylori-related changes on gastroscopy, or gastric biopsy findings. In order to exclude subjects with spontaneous regression or unintended eradication, classification of H. pylori-naïve stomachs was based on the following criteria: (1) no previous diagnosis of H. pylori infection, (2) negative findings on invasive and noninvasive H. pylori tests, (3) absence of gastric corpus atrophy (serum PG I ≤70 ng/mL and PG I/II ratio≤3), (4) absence of gastric atrophy, intestinal metaplasia, neutrophil, or H. pylori in the biopsied specimen (only a mild degree of mononuclear cell infiltration was permitted), and (5) regular arrangement of collecting venules on gastroscopy without evidence of past infection (either the presence of metaplastic gastritis, gastric xanthoma, or advanced atrophic gastritis ≥closed-type 2) [13].
Interpretation of H. pylori serologic test
Seropositivity was diagnosed when anti-H. pylori IgG level was ≥12.0 AU/mL, with 100% sensitivity and 75% specificity based on our previous study [14]. Seroconversion was considered when a seronegative subject showed seropositive test findings in the follow-up tests. The subjects were finally classified into subjects without seroconversion (persistent seronegative group) and subjects with seroconversion (seropositive group).
Primary outcome measurement
Accurate detection of H. pylori infection was considered the primary endpoint of this study. Current infection was diagnosed based on a positive Giemsa staining finding. Seronegative subjects with positive Giemsa staining findings were excluded, since non-H. pylori Helicobacter or Campylobacter may show false-positive Giemsa staining findings [15]. In these cases, follow-up H. pylori tests (i.e., 13C-urea breath test [UBT] with the aid of the POCone® [Otsuka Electronics Co., Ltd., Osaka, Japan]) were performed for confirmation to verify new infection in H. pylori-naïve subjects and to identify reinfection in subjects with past infection. Recrudescence was not considered in this study since all subjects with a history of successful eradication showed seronegative results for ≥24 months after eradication of the remote H. pylori infection.
Secondary outcome measurement
Seroconversion in follow-up tests was considered as the secondary endpoint. Seroconverted subjects without evidence of ongoing H. pylori infection were regarded as having false-positive test findings. The absence of infection after seroconversion was confirmed either by a negative 13C-UBT result or by spontaneous seroreversion of H. pylori with persistent RAC in follow-up tests.
Statistical analysis
Differences between the two groups were analyzed using the Student’s t-test for continuous variables and the chi-square test for categorical variables. Continuous variables with symmetrical distribution are presented as mean±standard deviation, and categorical variables are presented as the number of subjects with proportion (%). For continuous variables with asymmetrical distribution, data were presented as medians with ranges using the Kruskal-Wallis test. The Fisher’s exact test was used for categorical variables with an asymmetrical distribution. Differences between the initial and follow-up tests were analyzed using the Wilcoxon signed-rank test. All statistical analyses were performed using PASW statistics (version 24.0; SPSS Inc., Chicago, IL, USA). Statistical significance was set at p<0.05.
RESULTS
Characteristics of the included H. pylori-seronegative subjects
A total of 407 seronegative subjects underwent follow-up tests using the same serum anti-H. pylori IgG Chorus assay. Baseline characteristics and test findings are summarized in Table 1.
Incidence of seroconversion
During the mean follow-up of 57.7±21.4 months, 61 (15.0%) of 407 seronegative subjects showed seroconversion (Fig. 1). The annual seroconversion rate was 3.3%. The seroconverted subjects had a longer follow-up period (68.6±23.8 months vs. 55.8±20.4 months, p<0.001) and a higher initial serology titer than those without seroconversion (6.7±1.6 AU/mL vs. 5.7±1.3 AU/mL, p<0.001).
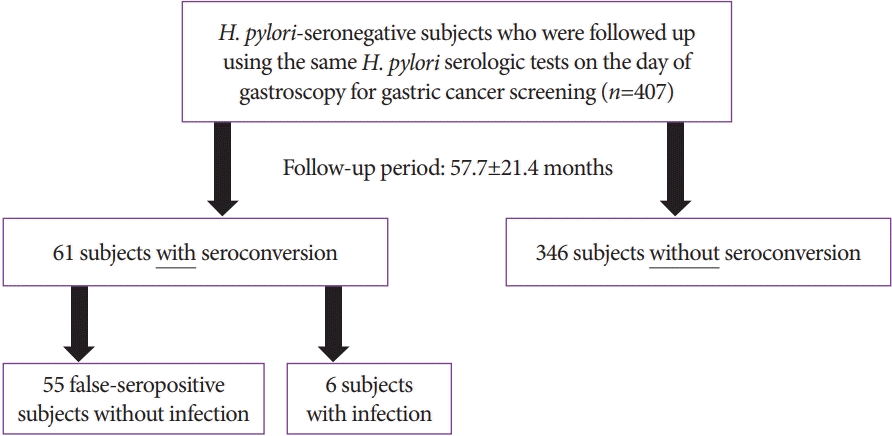
Flowchart of this study. A total of 407 consecutive Helicobacter pylori (H. pylori)-seronegative subjects were included in our study. During the mean follow-up period of 57.7±21.4 months, 61 subjects showed seroconversion; meanwhile, seronegative results persisted in 346 subjects. H. pylori infection was observed in six (9.8%) of 61 seroconverted subjects.
Seropositive subjects with H. pylori infection in the follow-up tests
H. pylori infection was detected in six (9.8%) of 61 seroconverted subjects. Therefore, H. pylori infection was observed in 2.0% (0.42% per year) of seronegative subjects during screening for gastric cancer. The initial and follow-up test results of the six infected subjects are summarized in Table 2.

Initial and Follow-Up Test Findings of the Six Seroconverted Subjects with Helicobacter pylori Infection
Changes in endoscopic findings were observed in an H. pylori-naïve subject with new infection (Fig. 2), as well as in subjects with reinfection in whom eradication therapy was previously performed (Fig. 3), and in a subject with unintended eradication (Fig. 4). Compared to the initial endoscopic findings, all infected subjects demonstrated diffuse red fundal appearance. The total Kyoto classification scores (p<0.001) and diffuse red fundal appearance scores (p<0.001) increased significantly.

Development of diffuse red fundal appearance on follow-up endoscopy in an Helicobacter pylori (H. pylori)-naïve subject with new infection. (A) Initial endoscopic findings of the fundus and cardia. The Kyoto classification score was 0 (A0, IM0, H0, N0 DR0). Serum pepsinogen (PG) and serology assays showed normal results, as summarized in Table 2. (B) Findings of follow-up endoscopy performed after four years. Multiple tiny hemorrhagic spots were observed in the fundus with positive Giemsa staining. The Kyoto classification score increased from 0 to 2 (A0 IM0 H0 N0 DR2). Seroconversion in H. pylori antibodies (6.4 → 21.7 AU/mL) was reported with higher serum PG I (69.5 → 85.1 ng/ml) and PG II (18.9 → 22.5 ng/ml) levels compared to the initial test findings.

Changes in the endoscopic findings of a subject who had a history of successful eradication four years prior to the initial visit. (A) Endoscopic findings of the fundus at the initial visit. The Kyoto classification score was 3 (A1 IM2 H0 N0 DR0). Successful eradication was confirmed by negative 13C-urea breath test findings. (B) Findings of follow-up endoscopy performed after six years. The development of diffuse red fundal appearance and positive Giemsa staining were observed. The Kyoto classification score increased from 3 to 5 (A1 IM2 H0 N0 DR2) with seroconversion (9.4 → 161.5 AU/mL). The serum pepsinogen (PG) I (46.4 → 97.2 ng/ml) and PG II (10.3 → 29.8 ng/ml) levels were higher compared to the initial test results, whereas the PG I/II ratio was lower (4.5 → 3.3).
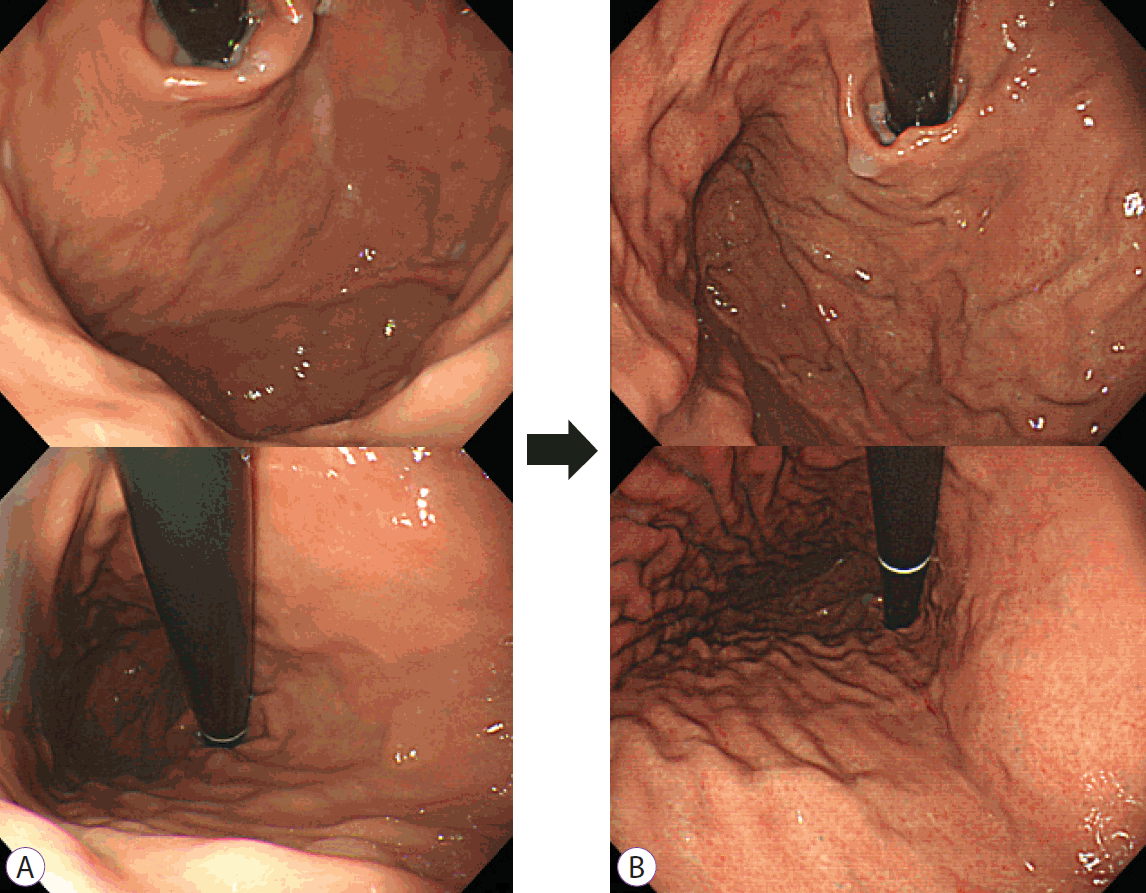
Findings of initial and follow-up endoscopy in a subject with unintended eradication prior to the initial visit. (A) Initial endoscopic findings of the fundus. Unintended eradication of Helicobacter pylori was considered in this study since intestinal metaplasia was observed in the biopsied specimens obtained from the antrum. Serum pepsinogen and serology assays showed normal findings, and the Kyoto classification score was 1 (A0, IM1, H0, N0, DR0). (B) Follow-up endoscopy performed after four years revealed diffuse red fundal appearance and enlarged gastric pit openings in the fundus. Positive Giemsa staining results were obtained with seroconversion (<5 → 158.1). The Kyoto gastritis classification score increased to two (A0 IM1 H0 N0 DR1).
False-seropositive subjects in the follow-up tests
Compared to the initial serologic titers, follow-up serologic titers showed a significant increase from 6.4 AU/mL to 22.0 AU/mL (p<0.001). However, there were no significant changes in the serum PG assay findings (Table 3). A small increase was observed in the Kyoto classification scores for gastritis (from 0.13 to 0.30, p=0.006) and metaplastic gastritis scores (from 0.11 to 0.27, p=0.006); however, none of these false-seropositive subjects exhibited a positive H. pylori test result in follow-up tests. 13C-UBT was performed in nine subjects, and it was negative in this group of subjects. Furthermore, 52 of 55 false-seropositive subjects showed persistent RAC on follow-up endoscopy. Spontaneous seroreversion of H. pylori was observed in 31 subjects who underwent the same serological follow-up procedure during screening for gastric cancer.
Difference between the follow-up test findings in the infected and false-seropositive subjects
The median serologic titer increased from 6.5 AU/mL to 132.7 AU/mL in the seropositive subjects with H. pylori infection (p=0.021). Hence, the serology titer was significantly higher among the infected subjects than the false-seropositive subjects (132.7 AU/mL vs. 22.0 AU/mL, p<0.001) at the time of seroconversion (Table 3). Moreover, serum PG II levels were higher in the infected group than in the false-seropositive group (22.8±10.5 ng/ml vs. 10.4±5.3 ng/ml, p<0.001), and serum PG I/II ratios were lower in the infected group than in the false-seropositive group (3.7±1.1 vs. 6.2±1.6, p=0.002).
In the follow-up endoscopies, the infected subjects showed a significant increase in the Kyoto classification scores (2.75 vs. 0.30, p<0.001), chronic atrophic gastritis scores (0.60 vs. 0.04, p<0.001), metaplastic gastritis scores (1.00 vs. 0.27, p<0.001), and diffuse red fundal appearance scores (1.33 vs. 0, p<0.001) (Table 3). All infected subjects had diffuse red fundal appearance; meanwhile, this finding was not observed in any of the false-seropositive subjects (p<0.001).
DISCUSSION
In this study, we found that only 9.8% of the seroconverted subjects were accurately diagnosed with H. pylori infection. Although seroconversion occurred annually in 3.3% of the seronegative subjects during screening for gastric cancer, the majority (90.2%) of the subjects showed false-positive results without infection. A new H. pylori infection was observed in only 2.0% of the seronegative subjects during the mean follow-up period of 58 months (0.42% per year). All infected subjects showed diffuse redness in the fundus; however, it was not observed in any of the false-seropositive subjects. Therefore, diffuse redness in follow-up endoscopies could be considered an indicator for accurate detection of infection.
The seroconversion rate was related to a higher initial H. pylori titer and a longer follow-up period. Our findings are in accordance with those of a recent study that reported a link between high negative titers and the risk of gastric cancer based on the degree of atrophic gastritis [16]. Moreover, follow-up serologic titers were useful for identifying infected subjects in this study. The serologic titers were significantly higher in the infected subjects than in the false-seropositive subjects. These findings are consistent with a recent study that demonstrated the usefulness of serum anti-H. pylori IgG titers for detection of reinfection [17]. In summary, a high-negative IgG titer warrants serologic screening due to the higher risk of seroconversion and gastric cancer. Meanwhile, an increase in serologic titers in follow-up tests is indicative of ongoing H. pylori infection.
In this study, it was beneficial to include the results of the serum PG assay, as well as those of the serological tests. The serum assay was useful in discriminating subjects with H. pylori-infection from false-seropositive subjects. Increased serum PG II levels and serum anti-H. pylori IgG titers, as well as decreased PG I/II ratios, were observed in the infected subjects in follow-up tests. Previous studies have shown that increased PG II levels are more specific for detecting active H. pylori infection than PG I levels [18], and PG I levels increase frequently due to drugs, including aspirin [19]. Differences in serum PG values between the eradicated and non-infected subjects were not detected at ≥24 months after successful eradication [20]. High serum PG I and PG II levels indicate hyperchlorhydric stomachs [21], whereas low serum PG I levels and PG I/II ratios indicate hypochlorhydric stomachs with extensive atrophy [22]. Consistent with these studies, PG II levels were significantly higher in infected subjects than in false-seropositive subjects; hence, the PG I/II ratio was significantly lower in infected subjects in this study. Serum assays cannot replace endoscopy for gastric cancer detection in a seroprevalent population; however, they are useful in discriminating between subjects at high-risk for diffuse-type gastric cancer (i.e., high-seropositive IgG titers with high PG II levels) and those with intestinal-type gastric cancer or gastric adenoma (i.e., low PG I levels with low PG I/II ratios), neuroendocrine tumors (i.e., extremely low PG I levels in patients with autoimmune gastritis, or high PG I levels in patients with Zollinger-Ellison syndrome), and H. pylori-negative gastric cancers [23].
Another notable finding of our study was diffuse red fundal appearance in infected subjects during follow-up endoscopy. Diffuse red fundal appearance represents the presence of infection, which progressively decreases after successful eradication [24]. Increased hemoglobin index of the fundic mucosa during active inflammation appears to induce the diffuse red fundal appearance, since active inflammation due to H. pylori occurs more aggressively in the corpus than in the antrum [25]. A recent study using 13C-UBT showed that the endoscopic Kyoto classification of gastritis was useful for diagnosing H. pylori infection, even in seronegative subjects with high serology titers [26]. Furthermore, the Kyoto classification scoring system was useful in predicting reinfection in subjects in which the infection had been previously eradicated [27]. The mean Kyoto score was 4.63 in subjects with active H. pylori infection [28]; meanwhile, it was close to 0 in non-infected subjects in previous studies [29]. The latter study showed that the endoscopy-based Kyoto score could detect false-seropositive subjects without infection, since the Kyoto score was 0 in 63.2% of the seropositive subjects with low seropositive titers. Consistent with these studies, infected subjects in our study showed a significant increase in the Kyoto classification scores at follow-up endoscopies. The development of diffuse red fundal appearance and positive Giemsa staining were detected in infected subjects, whereas none of these findings were observed in subjects with false-seropositive results.
There are some limitations in our study. For example, H. pylori infection was diagnosed based on positive Giemsa staining in seropositive subjects, and 13C-UBT was performed only in subjects who required confirmation. Most false-seropositive results were confirmed by spontaneous seroreversion and persistent RAC observed in follow-up endoscopies. Also, although image-enhanced endoscopy is useful for discriminating between H. pylori-active and inactive gastritis, it was not routinely performed in all screening endoscopies [30]. Despite these limitations, we observed a diffuse red fundal appearance with higher Kyoto classification scores, H. pylori serology titers, and PG II levels in the infected subjects compared to the false-seropositive subjects.
In conclusion, seroconversion occurred in 3.3% of the seronegative subjects annually; however, only 9.8% were accurately diagnosed with H. pylori infection. The majority (90.2%) of the seroconverted subjects showed false positive results with lower serology titers, lower serum PG II levels, and higher PG I/II ratios compared to the infected subjects. Moreover, spontaneous seroreversion and RAC were observed in false-seropositive subjects; meanwhile, a significant increase in the Kyoto classification scores for gastritis was observed in the infected subjects due to a diffuse red fundal appearance. Active H. pylori infection should be considered in the presence of diffuse red fundal appearance during screening for gastric cancer.
Notes
Conflicts of Interest: The authors have no potential conflicts of interest.
Funding
This study was supported by the National Research Foundation (NRF) of Korea, funded by the Ministry of Education (NRF2016R1D1A1B02008937).
Author Contributions
Conceptualization: Sun-Young Lee
Data curation: Young Jung Kim, SYL, Jeong Hwan Kim, In-Kyung Sung, and Hyung Seok Park
Formal analysis: YJK, SYL
Funding acquisition: SYL
Investigation: YJK, SYL, JHK, IKS, HSP
Methodology: TJK, SYL, JHK, IKS, HSP
Project administration: SYL
Resources: YJK, SYL, JHK, IKS, HSP
Software: SYL
Supervision: SYL
Validation: YJK, SYL, JHK, IKS, HSP
Visualization: YJK, SYL, JHK, IKH, HSP
Writing-original draft: YJK
Writing-review&editing: SYL