Endoscopic Management of Staple Line Leak after Bariatric Surgery: Surgeon’s Perspective
Article information
Abstract
Laparoscopic sleeve gastrectomy (LSG) has become a standalone primary procedure as a bariatric metabolic surgery since the early 2000s. The overall complication rate of LSG is reported to range from 2% to 15%. Staple line leakage (SLL) remains a major adverse event and occurs in approximately 1–6% of patients. Choosing the optimal treatment modality is a complex process. Clinicians must understand that nutritional support and drainage of fluid collection are essential for initial management. Conservative endoscopic management and sufficient drainage can resolve approximately 70% of SLLs. Endoscopic management of bariatric complications has been rapidly evolving in recent years and can be considered in all patients who are hemodynamically stable. We will review the available endoscopic management techniques, including stent placement (self-expanding stents and bariatric-specific stents), clipping, tissue sealant application, and internal drainage (double-pigtail stents [DPS] placement, endoscopic vacuum therapy, and septotomy). Stent placement remains the mainstream treatment for SLLs. However, healing with stents requires multiple sessions/stents and a long course of recovery. Endoscopic internal drainage is gaining popularity and has the potential to be a superior method. The importance of early intervention and combined endoscopic methods should be recognized.
INTRODUCTION
Laparoscopic sleeve gastrectomy (LSG) has become a standalone primary procedure as a bariatric metabolic surgery since the early 2000s [1]. Initially, it had been introduced as a first-step procedure to biliopancreatic diversion or duodenal switch in morbidly obese patients. Over time, it has gained popularity due to the relatively less demanding surgical technique, preservation of the natural anatomy, and a lower complication rate than that of other precedent procedures such as Roux-en-Y gastric bypass (RYGB) [2]. The overall complication rate of LSG is reported to range from 2% to 15% [3]. Complications include leakage, bleeding, and stricture.
Among all complications, staple line leakage (SLL) remains a major adverse event with increased morbidity and mortality [4]. SLL occurs in approximately 1–6% of patients and can develop into chronic fistulas. Most SLLs (approximately 85% of all cases) occur in the gastroesophageal junction [3]. The cause of SLLs can be either mechanical or ischemic. In both scenarios, the intraluminal pressure of the sleeve exceeds the tissue and staple line resistance leading to dehiscence of the staple line. It is generally believed that leaks presenting within the first 2 days of surgery are caused by mechanical causes (distal stenosis, tension along the staple line, tissue trauma, and hematoma) and technical failure in choosing the optimal staple height for the tissue thickness. Meanwhile, leaks caused by ischemia present >5–6 days after surgery [5]. Rosenthal et al. [6] categorized SLLs into acute (within 7 days), early (within 1–6 weeks), late (after 6 weeks), and chronic (after 12 weeks). The management of SLLs differs according to the time of diagnosis from the time of surgery, which will be further discussed below.
The diagnosis of SLLs can be difficult. Tachycardia with a heart rate of >120 bpm may be the only clinical presentation in many cases. Gastrointestinal series or computed tomography (CT) may assist in the diagnosis but can produce false-negative results, and must not delay return to the operating room (the intention of the authors was that the performance of the CT scan itself should not delay the decision for emergency operation) if the patient is hemodynamically unstable [7].
Choosing the optimal treatment modality for a patient with SLL, whether it be surgery or endoscopic management, is a complex process. However, clinicians must understand that the use of broad-spectrum antibiotics, proton pump inhibitors, nutritional support, and draingae of fluid collection are all essential for initial management [8]. The surgical options for chronic and refractory leak after LSG include conversion to RYGB, total or proximal gastrectomy (which may entail an esophagojejunostomy), or a Roux-en-Y fistulojejunostomy. However, reoperation has a high rate of morbidity and mortality in the setting of active intra-abdominal contamination [9]. Conservative endoscopic management and sufficient drainage can resolve approximately 70% of SLLs [10]. Endoscopic management of bariatric complications has been rapidly evolving in recent years and can be considered in all patients who are hemodynamically stable. We will review the available endoscopic management techniques, including stent placement (self-expanding stents and bariatric-specific stents), clipping, tissue sealant application, and internal drainage (double-pigtail stents [DPS] placement, endoscopic vacuum therapy [EVT], and septotomy).
STENT PLACEMENT
Endoscopic stenting is a minimally invasive procedure that allows early ambulation, enteral feeding, and greater patient comfort [11]. Fully covered self-expanding metal stents (SEMSs) are preferred for the management of SLLs. SEMSs have been the subject of a few meta-analyses in recent years. The success rate has been improving, and the most recent meta-analysis reported a success rate of 92% [10]. The most common and frustrating complication is stent migration. The migration rate can range from 23% to 59% [10,12]. Migration requires repeated procedures for repositioning, and migration into the small bowel necessitates surgical intervention. Other common complications include nausea, vomiting, reflux, and discomfort, which may lead to intolerance.
Recently, bariatric-specific stents have been introduced into the market. Some examples include the MEGA stent (an ultra-sized fully covered stent; Taewoong Medical, Gimpo, Korea), BETA stent (with two migration cuffs; Taewoong Medical), and GASTROSEAL stent (MITECH, Seoul, Korea) [12]. The success rate of the MEGA stent was reported to be 82%. The migration rate was 18%, which is considerably lower than that of standard SEMSs. The number of procedures and stents required per patient was 3 and 1.3, respectively [13]. These numbers are significantly lower than those for standard SEMSs. However, pain, vomiting, and excessive salivation were observed in all patients. Patient education and assurance are important for preventing intolerance and improving outcomes.
A few additional points need to be addressed. The endoscopist must remember that drainage, absence of distal obstruction, and destruction of any epithelization of the fistula tract must be achieved along with the main procedure. Oral intake is routinely withheld for 24–48 h for full stent expansion, and a liquid to semi-solid diet can be tolerated for the duration of stenting. Plain radiography can be performed every 1–2 weeks to confirm the stent location. Any change in symptoms (pain intensity, fever, or vomiting) or recurrence of symptoms should be addressed, as this may suggest stent migration [11]. The duration of stent dwelling varies greatly. However, most authors recommend 6–8 weeks [10]. Earlier intervention, SLLs <10 mm, no history of previous gastric banding, and first endoscopy were all reported to be associated with better success of endoscopic treatment [14]. After the optimal 8 weeks of stenting, an endoscopic evaluation should be performed to assess the healing state. Gastrografin injection into the presumed leakage site is performed after the removal of the current stent. The endoscopist should be prepared for immediate reinsertion of another stent in cases of dye leakage during endoscopy. If there is evidence of leakage, the next endoscopy can be considered 4 weeks after reinsertion of another stent (the removed stent cannot be reinserted and the authors wanted to stress this point).
CLIPPING
The over-the-scope-clip (OTSC; Ovesco Endoscopy, Tübingen, Germany) system is a relatively novel clipping system that has been reported to successfully treat gastric leaks and fistulas [13]. It can be applied for the treatment of SLLs <10 mm [15]. A systematic review published in 2017 reported a success rate of 86.3% [16]. However, this study included cases with additional or concomitant endoscopic procedures. In a recent meta-analysis, the success rate of clipping alone was 67%. This further highlights the importance of combined endoscopic methods [10]. Considering the low success rate of the OTSC system alone in SLLs and its cost, a paper published in 2020 concluded that the OTSC system should be considered an option only for patients who could not tolerate other endoscopic treatments [17]. The OTSC system is also inappropriate for chronic fistulas with difficult-to-approximate fibrotic tissue [18]. Complications related to clipping (anchor migration, tear, etc.) are rare [10].
TISSUE SEALANT APPLICATION
Tissue sealants include fibrin glue and cyanoacrylate. Fibrin glue is the most commonly used sealant for fistula closure, and its application after various operations, including LSG, biliopancreatic diversion, and total gastrectomy, has been reported [19]. In addition to occluding the defect itself, fibrin glue also plays a role in wound healing by inducing cellular response to tissue damage and promoting neovascularization and fibroblast proliferation by forming matrix-building strands [20]. The current success rate ranges from 92.8% to 100% [10], although most cases require repeated sessions for complete closure. The main factors for failure are a large orifice or non-compliance of the patient to the scheduled application. The main advantages of fibrin glue are its easy application, low cost, and lack of severe complications. Brushing with a cytology brush has been recommended to clean debris and granulation tissue from the fistula orifice before sealant application [21]. Overall, complications related to fibrin glue are rare. In one study, 12.5% patients presented with pain and fever after fibrin glue injection [21].
Cyanoacrylate is a synthetic glue that functions as a mechanical sealant with high adhesive and antibacterial properties. It is six times less expensive than fibrin glue. Its efficacy has been demonstrated to be as high as 96.8% in various anastomotic leakages [22]. However, its application has been limited to date because of its difficult handling and delivery, as well as potential proinflammatory effects [10].
INTERNAL DRAINAGE
Achieving adequate drainage in the treatment of SLLs is paramount to success. When proper drainage is not initially performed, SLLs may recur and develop into chronic fistulas even with a long duration of treatment with other endoscopic modalities such as SEMS placement. DPS placement, EVT, and septotomy are discussed below in more detail.
Double-pigtail stents placement
The DPS is a useful option for the drainage of collected fluids into the sleeved stomach. This induces the collapse of the abscess cavity, leading to closure of the SLL [12]. The drain itself induces reepithelialization and guides the healing of the fistula [23]. The reported success rate ranges from 79% to 95% when coupled with enteral nutrition [12,24]. The mean time to heal was reported to be 55.5 days, with 2.95 procedures required per patient [24]. This method should be applied to SLLs with a fistula orifice <10 mm and can be utilized with SEMSs in larger leaks [25]. Any external drains should immediately be removed once internal drainage is achieved to prevent the formation of an enterocutaneous fistula [12]. A complication rate of 13.7% has been reported, with the most severe complication being migration of DPS, which can lead to splenic parenchymal hemorrhage or abscess [26]. Most reports recommend at least 4 weeks of nasojejunal feeding. The most significant advantages of DPS are the minimal discomfort experienced by patients and the high success rate even in chronic SLLs.
Endoscopic vacuum therapy
EVT is becoming an increasingly popular tool for the management of SLLs. This is because of the ability of endoscopic intraluminal EVT to successfully maintain drainage of fluid collection, which is not possible with SEMSs [9]. The success rate was reported to be 89% in patients with SLL in whom drainage with SEMS failed [27]. EVT can be performed under conscious sedation or general anesthesia. Injection of botulinum toxin into the pylorus has been suggested as an adjunct therapy to reduce distal pressure and ensure better healing. Cases of stricture and hemorrhage of adjacent vascular structures in patients with erosive conditions have been reported as EVT-related complications [28]. Compared with EVT, DPS drainage requires the placement of two stents and carries the risk of migration with the subsequent complications mentioned above. However, it is relatively less invasive and does not require potential general anesthesia. Conversely, EVT can offer a considerably shorter treatment period than DPS placement because it induces healing without the formation of a chronic fistula.
Septotomy
Septotomy first appeared in the literature as stricturotomy for chronic fistulas after RYGB. This procedure enables internal drainage while allowing deviation of oral intake into the sleeved stomach [29]. The success rate was reported to be 80% in a small case series in 2020 and 100% in another case series in 2017 [18,30]. Argon plasma and needle knife as a cutting device have been used in previous reports, although any device with hemostatic technology can be applied. Septotomy requires multiple sessions and, accordingly, has also been called “progressive” endoscopic septotomy [30]. The entire cavity should be gradually exposed with each session by completely cutting the residual septum. The number of sessions required for healing was reported to range from 1.8 to 5 sessions per patient [18].
CONCLUSION
Stent placement remains the mainstream treatment for SLLs. However, healing with stents requires multiple sessions/stents and a long course of recovery. A management algorithm (Fig. 1) is provided as a reference for clinicians. Endoscopic internal drainage is gaining popularity and has the potential to be a superior method. The importance of early intervention and combined endoscopic methods should be recognized.
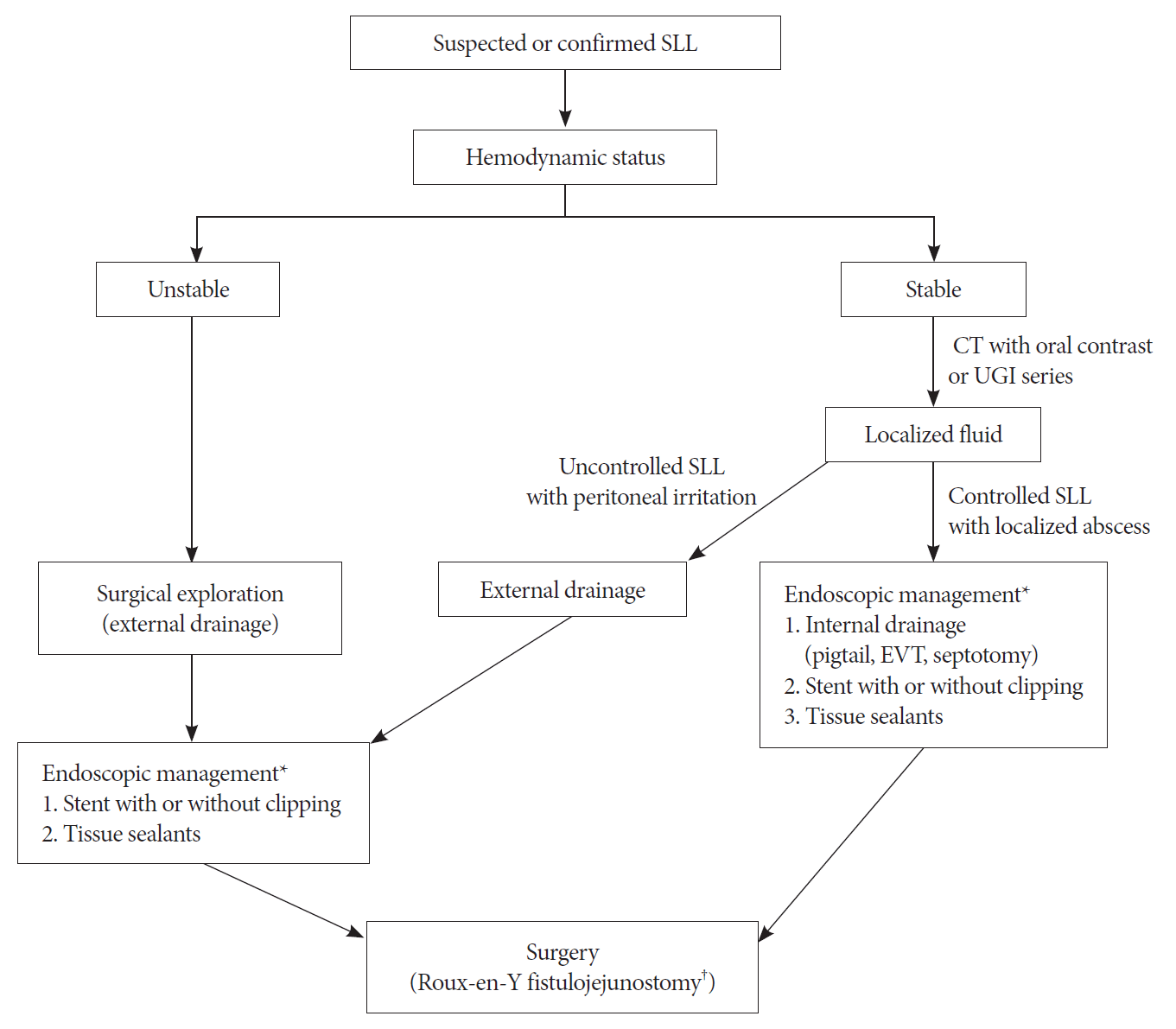
Management algorithm for staple line leakage. CT, computed tomography; EVT, endoscopic vacuum therapy; SSL, staple line leakage; UGI, upper gastrointestinal. *The procedures are recommended in the order of the item numbers. The method of internal drainage should be selected according to the experience and preference of the endoscopist. †Roux-en-Y fistulojejunostomy is the preferred surgical method.
Notes
Conflicts of Interest: The authors have no potential conflicts of interest.
Funding:None.
Author Contributions
Data curation: Dae Geun Park
Resources: DGP
Supervision: Yong Jin Kim
Writing-original draft: Yoona Chung
Writing-review&editing: YC, YJK
