The Additive Effect of Platelet-Rich Plasma in the Treatment of Actively Bleeding Peptic Ulcer
Article information
Abstract
Background/Aims
Peptic ulcer bleeding is the most common cause of upper gastrointestinal tract bleeding. Platelet-rich plasma (PRP) enhances tissue repair, and is therefore used in various medical treatments. A combination of mechanical or electrothermal hemostasis has been recommended for upper gastrointestinal tract bleeding treatment. This study evaluated the additive efficacy of PRP in bleeding peptic ulcer hemostasis and recovery.
Methods
Eighty patients with peptic ulcer bleeding were initially treated by hemoclipping, and were randomly chosen for either additional PRP (n=40) or additional epinephrine (n=40) injections. Both groups were compared with regard to achieving hemostasis and the frequency of complications.
Results
Hemostasis was immediately achieved in both groups. Two patients (5%) in the PRP group and 8 (20%) patients in the epinephrine group experienced rebleeding after 15.9±2.8 and 12.3±3.7 days, respectively. They were managed by PRP injection in addition to proton pump inhibitor infusion. Hemoglobin was substantially increased in the PRP-treated group with full recovery occurring in 60.5% compared to 31.3% of patients in the epinephrine group (p=0.001). There was no recurrent bleeding in the PRP group, but 4/32 (12.5%) patients in the epinephrine group exhibited rebleeding.
Conclusions
PRP showed additional benefit in reducing peptic ulcer bleeding with no reported significant complications. Clinical trial (NCT03733171).
INTRODUCTION
Many endoscopic maneuvers are used to induce hemostasis and minimize rebleeding from an actively bleeding ulcer. These include injecting the ulcer with chemical agents, such as tissue adhesive glue, fibrin, and epinephrine; coagulation by argon plasma or cryotherapy; or mechanical control using hemoclips and rubber bands [1].
Platelet-rich plasma (PRP) has gained interest in medicine due to its ability to hasten tissue repair [2]. This autologous subtype of plasma has powerful adhesive and hemostatic characteristics, having a higher platelet ratio than other tissue adhesives [3].
The platelets release several effector mediators that initiate healing, including vascular endothelial, epidermal, fibroblast, and connective tissue growth factors, like insulin-like growth factor-1 and transforming growth factor alpha and beta [4,5].
PRP provides cell adhesion molecules, mainly fibronectin and vitronectin, which are converted to a bioactive state that induces direct cellular growth, matrix deposition, and collagen production, thus inducing tissue regeneration [6].
PRP has been used in many fields of medicine, for many indications such as chronic tendinitis and osteoarthritis, and was found to be more effective in reducing pain than hyaluronic acid. It has also been used in patients with alopecia, pressure sores, dental surgery, colonic surgeries, and burns [7-11].
The current study aimed to explore the usefulness and possible curing effect of PRPs in bleeding peptic ulcer.
PATIENTS AND METHODS
The current work was carried out in the Endoscopy and Gastroenterology Division of the Zagazig Faculty of Human Medicine from September 2018 to April 2019. The patients were admitted for bleeding peptic ulcer (referred from other hospitals to our tertiary center for further endoscopic interference and management).
Exclusion criteria
We excluded patients with non-peptic ulcer bleeding, shock (diagnosed by an increased pulse rate >100 beats/min, systolic hypotension <100 mmHg, or both) until the patients were stabilized, coagulopathy, severe bleeding diathesis, anticoagulant therapy, cardiopulmonary compromise, uncontrolled systemic hypertension, recent ischemic heart disease, active arrhythmia, and patients who rejected participation.
Absolute contraindications
Severe thrombocytopenia (platelet count <50 ×103 /uL); hemodynamic instability; septicemia; use of drugs that affect platelet count, like analgesics, within 48 hours of the procedure, and steroid use within the preceding two weeks; fever; hematological malignancies; and irreversible shock.
Before the procedure, the patients or their relatives signed a consent form after understanding the benefits and possible risks of endoscopy, and other treatment options.
The study was approved by the Institutional Review Board of the Zagazig Faculty of Medicine (ZU-IRB-4940), and was posted on ClinicalTrials.gov (Identifier: NCT 03733171). All authors accessed and revised the study data and final manuscript.
Patient assessment
All the participants were approached and questioned, and they underwent careful clinical evaluation, laboratory assessment of blood elements, liver and kidney function tests, coagulation test, and cardiac assessment by electrocardiography. After hemodynamic stabilization with blood transfusion, if the hemoglobin level was below 7 g/L, upper gastrointestinal endoscopy was performed within 24 hr [12].
Criteria of acute bleeding was stratified by Forrest (F) classification
- Acute hemorrhage: Forrest Ia (spurting ulcer) and Ib (oozing surface).
- Signs of recent hemorrhage: Forrest IIa (visible vessel without bleeding), IIb (ulcer covered with adherent clot), and IIc (flat pigmented coffee ground base).
- Ulcers without active bleeding: Forrest III (fibrin-covered clean ulcer base).
The ulcer was categorized according to size as less than 2 cm or more than 2 cm [13]. About 1-2 mL of PRP or diluted epinephrine was applied all around the ulcer, along with mechanical compression, until the bleeding ceased [14,15]. PRP-treated patients were administered up to 4 repeated injections of PRP (each 1 mL), while the epinephrine group was treated with diluted epinephrine (1–2 mL of 1:10.000 adrenaline acid tartrate) [11]. Subsequently, both groups underwent hemoclipping.
Initial hemostasis was determined by blood leakage stopping after 3 min. IV infusion of proton pump inhibitors (PPIs) were then initiated at a loading dose of 80 mg, followed by administration of continuous infusion of 8 mg/hr for 3 days. The patients were given oral PPIs and were observed for 1 week before discharge [16].
Recurrent bleeding was confirmed if features of gastrointestinal bleeding were evident, including hematemesis, melena, hematochezia, fresh blood in the nasogastric tube, development of hemorrhagic shock (systolic hypotension <100 mmHg or tachycardia >100 beats/min, drop of hematocrit or hemoglobin levels by more than 5% or 2 g/dL) over a 24-hour period after initial resuscitation [17]; these patients were subjected to re-endoscopy after hemodynamic stabilization.
Permanent hemostasis was confirmed if features of recurrent GI bleeding were not evident during the 10-day period after initial hemostasis [17]; re-endoscopy was performed after one month of initial hemostasis, and then again after two months, to assess the ulcer healing.
Randomization
Single-blinded randomization was done, that was, the endoscopists knew the treatment allocation, but the patients did not. The patients were given the assumed treatments randomly (1:1 proportion), determined using sealed envelopes with the predefined therapeutic endoscopic approach arranged via a computer-assisted random table. Every patient received a randomly selected envelope.
Outcome assessment
The hemodynamic parameters were measured every 2 hr for the first 12 hr and every 4 hr for the next 36 hours. The hemoglobin and hematocrit values were evaluated daily and blood transfusion was given if the hemoglobin level was below 7 g/dL or if signs of shock were evident.
Rebleeding was suspected with the presence of deranged vital signs as mentioned before, persistence of melena, and drop in the hemoglobin level despite blood transfusion of at least 2 units, and emergency endoscopy was performed.
The primary outcome was recurring bleeding within 14 days or complete ulcer healing at 3 months, which was defined endoscopically as complete regeneration, while the secondary outcome was the need for surgery and all-cause mortality.
Platelet-rich plasma preparation method
Thirty milliliters of blood was collected to prepare the PRP sample 30 minutes before the second-look endoscopy, as the patients were referred to our tertiary center for further endoscopic management. Sodium dextrose was added to stop platelet activation. A soft spin (1st centrifugation at 300 g for 5 minutes) was given to obtain the supernatant plasma with platelets, which were then transferred to another sterile tube and centrifuged at a greater velocity (a hard spin at 700 g for 15 minutes) to obtain a platelet aggregate. The lower part was the PRP, which was 4–5 mL, and the upper part was platelet-free plasma. Immediately before endoscopic injection, 0.3 mL of 10% calcium chloride was added for every ml of PRP obtained.
Statistical analysis
Data were statistically evaluated using SPSS version 20 for Windows (IBM Co., Armonk, NY, USA). Results are presented as mean±standard deviation. Categorical variables were evaluated using the χ2 test, and continuous variables were compared using the Student’s t-test. A p-value <0.05 was considered significant.
RESULTS
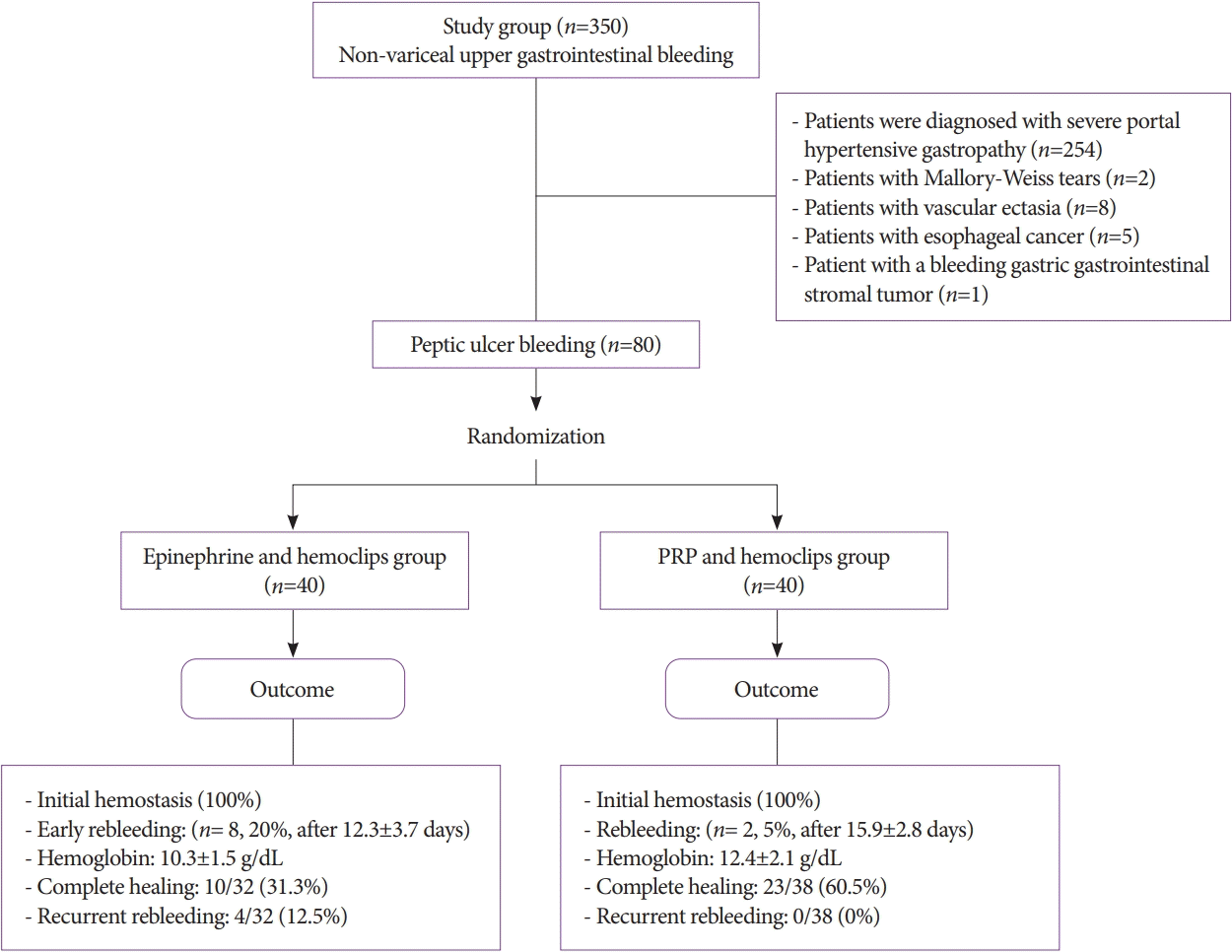
Three hundred and fifty patients presented with non-variceal upper gastrointestinal bleeding: 254 patients were diagnosed with severe portal hypertensive gastropathy, 2 patients with Mallory-Weiss tears, 8 patients with vascular ectasia, 5 patients with esophageal cancer, and 1 patient with a bleeding gastric gastrointestinal stromal tumor. Finally, patients with bleeding peptic ulcer were selected (n=80) and single-blindedly randomly stratified into PRP-treated patients (n=40) or epinephrine-treated patients (n=40) (Fig. 1).
Ulcers features in the platelet-rich plasma-treated group
Size: small (<2 cm; n=28, 70%), large (>2 cm; n=12, 30%). Site: duodenal bulb (n=10, 25%), gastric antrum (n=12, 30%), and corpus (n=18, 45%).
Ulcer type: Forrest Ia with spurting (n=12, 30%), and Forrest Ib with an oozing surface (n=28, 70%).
Epinephrine-treated group
Size: small (<2 cm; n=32, 80%), large (>2 cm; n=8, 20%) Site: duodenal bulb (n=5, 12.5%), gastric antrum (n=16, 40%), and corpus (n=19, 47.5%).
Ulcer type: Forrest Ia with spurting (n= 8, 20%), Forrest Ib with an oozing surface (n=32, 80%).
Outcomes
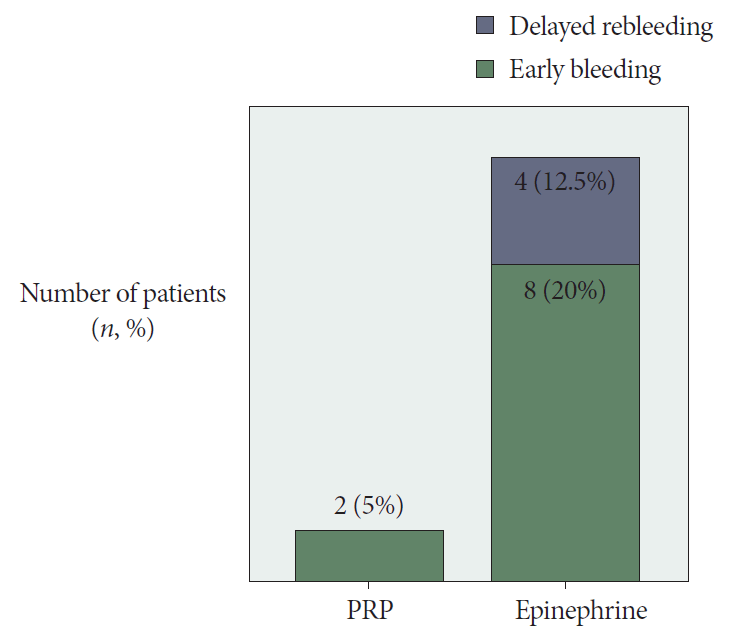
In both groups, hemostasis was immediately achieved. Two patients (5%) in the PRP group and 8 (20%) patients in the epinephrine group exhibited rebleeding after 15.9±2.8 days and 12.3±3.7 days, respectively, and they were managed by PRP injection in addition to PPI infusion with immediate hemostasis.
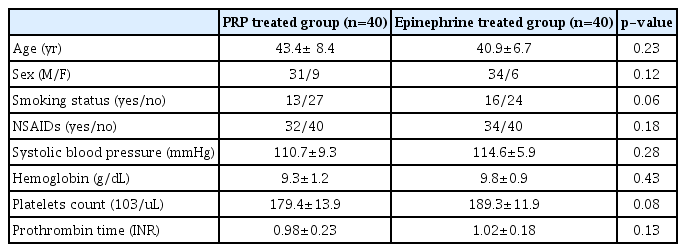
The demographic, hemodynamic, and laboratory features of both groups are shown in Table 1. The confounding variables such as age, sex, body mass index, and nonsteroidal anti-inflammatory drug intake showed a non-significant difference. The initial hemostatic response to both endoscopic interventions was comparable in both groups; however, the early rebleeding rate was considerably lower in PRP-treated patients, with an overall success of 95% (38/40), when compared to 80% (32/40) in the epinephrine group. This was possibly due to the tissue healing effect of PRP.
Hemoglobin was substantially increased in the PRP-treated group with full recovery in 60.5% patients compared to 31.3% in the epinephrine group (p=0.001) (Table 2, Fig. 2).
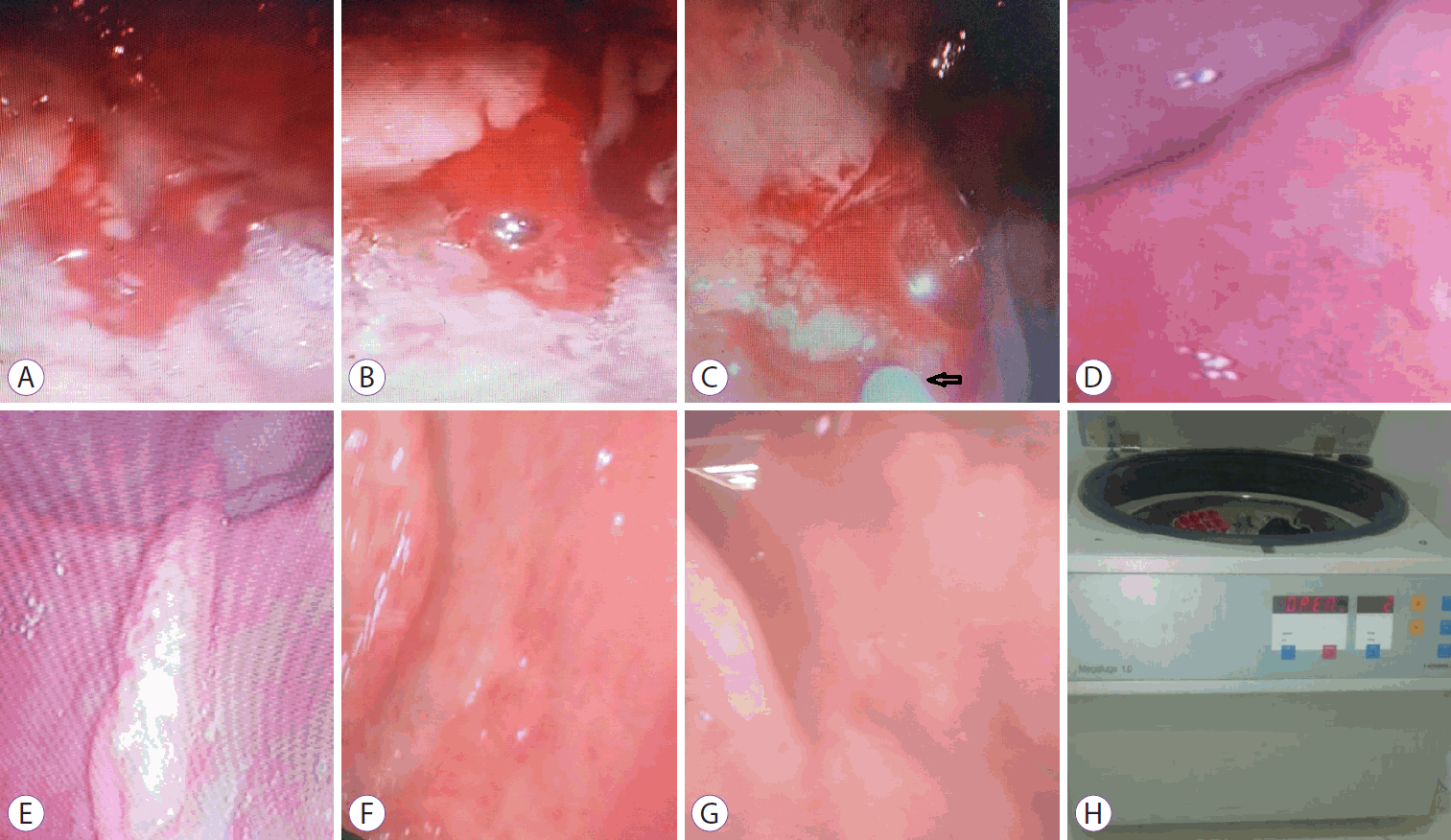
(A, B) Actively bleeding duodenal ulcer. (C) During injection of platelet-rich plasma (PRP). (D) Hemostasis after PRP injection. (E) Effect of PRP after 1 month. (F) Effect of PRP after 3 months. (G) Complete healing after 5 months. (H) The centrifugation device.
Three patients in the epinephrine-treated group developed therapy-related complications, such as arrhythmia, in the form of supraventricular tachycardia (n=2) and hypertension (n=1), with no reported allergic reactions or technical problems in both groups.
During the months of follow-up, recurrent bleeding was not experienced by PRP-treated patients; however, 4/32 (12.5%) (p=0.01) of epinephrine-treated patients exhibited rebleeding, and upper endoscopy revealed re-oozing from a partially healed peptic ulcer, which was controlled by PRP injection (Figs. 2 and 3).
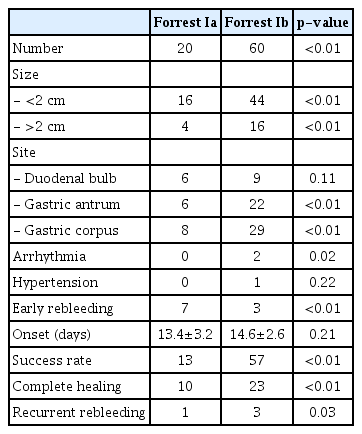
Table 3 shows the subgroup analysis of therapeutic outcomes according to the Forrest classification. Forrest Ib was significantly prevalent in both groups; arrhythmia and hypertension were predominant in Forrest Ib in adrenaline-treated patients and early rebleeding in both subgroups was significantly higher in Forrest Ia. In both groups, the success and complete healing rates were significantly higher in Forrest Ib. Table 4 shows the therapeutic outcomes according to the Forrest classification. Most patients were in Forrest Ib, with a size less than 2 cm, and mainly located in the gastric corpus. The success, complete healing, and recurrent rebleeding rates were significantly higher in patients in Forrest Ib; however, early rebleeding was more predominant in Forrest Ia.
DISCUSSION
This study is the first to compare the effectiveness of endoscopic therapy using PRP with one of the commonly applied methods to control peptic ulcer bleeding.
Acute upper gastrointestinal hemorrhage may have significant associated morbidity, with an incidence of nearly 150 cases per 100,000 people. The most common causes are peptic ulcers, mainly duodenal (35%) and gastric (20%) ulcers [18,19]. Endoscopic therapy, aiming to achieve hemostasis, is considered the mainstay treatment for bleeding peptic ulcers through injection of chemical or pharmacological agents, contact coagulation, or mechanical occlusion of the bleeding vessel. Among these therapeutic interventions, injection of epinephrine is the most used endoscopic method [20], primarily through establishment of local tamponade by the volume injected and local vasoconstrictive effects [17].
Recent practice guidelines recommend a combination of epinephrine with a second endoscopic hemostatic approach, such as mechanical therapy or thermal coagulation [21,22]. Epinephrine reduces or stops bleeding and improves the visual field for subsequent therapy. New alternative endoscopic modalities, such as topical hemostatic sprays, over-the-scope clips, and hemostatic forceps are available [23,24]. However, the recently introduced agents such as inert nanopowder (Hemospray®; COOK MEDICAL. Bloomington, IN, USA) [24], starch-derived polysaccharide hemostatic system (EndoClot® Polysaccharide Hemostatic System [PHS]; EndoClot Plus, Santa Clara, CA, USA), and the Ankaferd blood stopper [25,26] are expensive, and randomized controlled trials are needed to compare them with traditional hemostasis methods. In addition, they do not provide a permanent cure for the underlying cause.
PRP is considered a valuable source of platelets, growth factors, and cytokines that augments tissue healing and has been used in many indications, such as acute muscle strains, tendinopathy and osteoarthritis [27], androgenic alopecia, bed sores, wound healing, and anal fistula [28]. PRP induces hemostasis through local compression and the high content of bioactive cell adhesive proteins, which have an essential role in tissue repair, such as fibronectin, fibrin, and vitronectin [29]. Its biodegradability prevents allergic reactions, tissue damage, or fibrosis [30].
Platelets can stimulate the clotting and healing of the affected tissues. At the stage of platelet adhesion, platelet membrane receptors GPIIb and GPIIIa become activated and bind to fibrinogen and von Willebrand factor, fixing platelets to the site of tissue injury and forming a platelet plug by entrapping activated platelets into a mesh of fibrin strands, which adds strength to the formed clot. During the inflammation phase, macrophages and infiltrating neutrophils migrate to remove the red blood cells, hemoglobin, and bacteria, and the activated platelets release the contents of their alpha granules, mainly cytokines, growth factors, and bioactive proteins, which are needed for tissue repair and healing [31].
PRP and hemoclips could control bleeding from peptic ulcer in 95% of patients, which was superior to results obtained using diluted epinephrine and hemoclips (80%), with significantly lower rates of ulcer recurrence or rebleeding. PRP can be prepared easily and rapidly, without excessive expenditure (250 Egyptian Pounds, i.e., 15 $) and has no side effects. PRP has a compressing effect on the bleeding site and is sticky, it persists for a longer time, and can induce tissue healing. The most recent research supporting our results explored the impact of PRP on curing ulcers induced by endoscopic submucosal dissection and showed that local injection of PRP is a safe and effective procedure [32].
In the current study, Forrest Ib was more prevalent; however, significant early rebleeding was observed in Forrest Ia. The rates of success and complete healing were significantly higher in Forrest Ib. This was consistent with the results reported by Jensen et al., which showed that the risk for rebleeding was higher for Forrest Ia, Forrest IIa, and Forrest IIb lesions when compared with Forrest Ib lesions, with odds ratios of 6.7, 2.6, and 4.1, respectively [33].
The limitations of the current study are that it is a single center study and a multicenter study is necessary; however, PRP was efficient, cost-effective, and had no complications, bringing about sustained and complete healing of bleeding peptic ulcers.
Notes
Conflicts of Interest: The authors have no potential conflicts of interest.
Funding
None.
Author Contributions
Conceptualization: Waseem Seleem
Data curation: WS, Amr S Hanafy
Formal analysis: WS, ASH
Methodology: WS, ASH
Supervision: WS, ASH
Validation: WS, ASH
Visualization: WS, ASH
Writing-original draft: ASH
Writing-review&editing: WS, ASH
Acknowledgements
Appreciation to the Zagazig University main Lab for its support in this research.