A prospective study of fully covered self-expandable metal stents for refractory benign pancreatic duct strictures
Article information
Abstract
Background/Aims
Fully covered self-expanding metal stents (FCSEMSs) are a relatively novel option for treating painful main pancreatic duct refractory strictures in patients with chronic pancreatitis. Herein, we aimed to assess the efficacy, feasibility, and safety of FCSEMSs in this patient group.
Methods
This prospective single-center study included patients who underwent endoscopic retrograde pancreatography with FCSEMS placement. The primary endpoints were the technical and clinical success rates. A reduction in visual analog scale pain score of >50% compared with that before stent placement was defined as clinical success. Secondary endpoints were resolution of pancreatic strictures on fluoroscopy during endoscopic retrograde pancreatography and the development of stent-related adverse events.
Results
Thirty-six patients were included in the analysis. The technical success rate was 100% (n=36) and the clinical success rate was 86.1% (n=31). There was a significant increase in stricture diameter from 1.7 mm to 3.5 mm (p<0.001) after stent removal. The mean visual analog scale pain score showed statistically significant improvement. At 19 months of follow-up, 55.6% of the patients were asymptomatic. Stent migration (16.7%), intolerable abdominal pain (8.3%), development of de novo strictures (8.3%), and mild pancreatitis (2.8%) were the most common adverse events.
Conclusions
FCSEMS placement showed good technical and clinical success rates for achieving pain relief in patients with refractory main pancreatic duct strictures.
INTRODUCTION
Chronic pancreatitis (CP) is an ongoing and long-standing inflammatory disease of the pancreas characterized by irreversible structural changes leading to frequent or constant pain episodes and/or a permanent loss of function. Pain is the most common symptom in patients with CP. Strictures in the head or body of the main pancreatic duct (MPD) are a complex adverse event in the natural history of CP. Such a stricture causes pancreatic duct hypertension with subsequent continuous pain, which is often unresponsive to standard medical therapy alone.1 The reported incidence of such strictures is 79%.2
In patients with painful CP, the placement of one plastic stent (PS) across the MPD stricture is the treatment of choice.3,4 Various studies have reported pain relief in 57% to 75% of patients after PS placement.5-8 However, definitive stent removal is challenging in 30% to 40% of patients due to persistent stricture and subsequent pain.2,9-12 In such refractory cases, stricture resolution and the prevention of pain recurrence can be achieved by multiple PS placement or fully covered self-expanding metal stent (FCSEMS) placement.13-18 In the present study, we aimed to assess the efficacy, feasibility, and adverse events associated with FCSEMS placement in CP patients with refractory MPD strictures.
METHODS
Patients
This prospective single-center study of patients with painful CP was performed in the largest tertiary care hospital in Eastern India. Consecutive patients aged >18 years who were diagnosed with chronic pancreatitis, persistent pain, and refractory stricture in the pancreatic head or body region and initially treated with pancreatic sphincterotomy and PS insertion were included. The diagnosis of CP was based on morphological findings by magnetic resonance cholangiopancreatography or endoscopic ultrasound and confirmed by endoscopic retrograde pancreatography (ERP). Painful CP was defined as three or more episodes per year of a pancreatic-type pain, specifically pain in the epigastric region that radiated to the back and/or was relieved upon leaning forward. Exclusion criteria included the following: previous pancreatic surgery, acute pancreatitis requiring hospitalization, patient denial, papillary inaccessibility with a duodenoscope, associated pancreatic pseudocyst and/or walled-off necrosis, and suspected pancreatic cancer diagnosed during follow-up. Data collection included details on demographics; alcohol consumption; pain severity as assessed by the visual analog scale (VAS); procedure-related adverse events; and follow-up data.
Endoscopic FCSEMS treatment
All procedures were performed under propofol sedation with or without endotracheal intubation with the patient in the prone position under continuous monitoring. Three experienced investigators followed a standardized protocol using a standard duodenoscope (TJF-180V; Olympus Medical Systems Co., Ltd., Tokyo, Japan). Extracorporeal shockwave lithotripsy (ESWL) was performed to fragment the stones prior to ERP in cases of stones measuring >5 mm in the MPD head or body region on imaging. After cannulation of the major or minor papilla during ERP, a hydrophilic guidewire (0.020 or 0.035 inch) (Radiofocus; Terumo Europe NV, Leuven, Belgium/Jagwire Super Stiff; Boston Scientific, Galway, Ireland) was inserted into the MPD. All patients underwent MPD stricture dilation using a 4–6-mm-diameter hydrostatic balloon catheter (Titan; Boston Scientific). In cases of difficult strictures, hydrostatic balloon dilatation was not possible and dilatation was performed using a Soehendra stent retriever (Wilson-Cook Medical Inc., Limerick, Ireland). Pancreatic stones were extracted whenever possible using a Dormia basket (Olympus Medical Systems Co., Ltd.). FCSEMSs were then deployed across the stricture over the guidewire. An FCSEMS of 6 mm or 8 mm in diameter was selected according to stricture length tailored from the pancreatic sphincter orifice. Niti-S (Bumpy) stents (Taewoong Medical, Seoul, Korea) were used.
End points
The primary endpoints were technical and clinical success. Secondary endpoints were resolution of the pancreatic stricture on fluoroscopy during ERP and adverse events related to the FCSEMS, such as migration, occlusion, severe rise in pain post-stenting, de novo MPD stricture, and pancreatic sepsis.
Technical success was defined as the exact endoscopic positioning of the FCSEMS along the stricture length with free flow of the contrast material through it. Pain scores and analgesic requirements were collected before and after the procedure over the phone or during the office visit. Pain scores were recorded as VAS, if available at each office visit. A reduction in VAS score of >50% versus that before stent placement was defined as clinical success. Stricture resolution was defined as the passage of an inflated extraction balloon across the stricture at the time of stent removal and an increase in stricture diameter during the follow-up ERP.
Stent migration was defined as FCSEMS movement above or below the stricture. During FCSEMS placement or retrieval, the development of a clinical infection was defined as pancreatic sepsis. New pancreatic ductal stricture development at the end of the FCSEMS was defined as de novo stricture development.
Follow-up
Follow-up appointments were scheduled for 1, 3, and 6 months after stent placement. Patients who developed adverse events, such as abdominal pain, underwent abdominal computed tomography. In cases of pain, relapsed pancreatitis, or any stent-related adverse events, ERP was considered. Stent removal was performed 6 months after stent placement. Follow-up after stent removal was planned whenever adverse events (abdominal pain) occurred or every 3 months, whichever occurred earlier.
Statistical analysis
Categorical parameters are expressed as frequencies and proportions and continuous variables as medians with interquartile ranges (IQRs). Changes in MPD stricture diameter (measured before stent placement and after stent removal) and changes in VAS pain scores (before and after stent placement) were compared using Wilcoxon signed-rank test. All statistical analyses were performed using IBM SPSS software (ver. 18.0; IBM Corp., Armonk, NY, USA).
Ethical statements
The Institutional Review Board (IRB) of Apollo Multispecialty Hospitals (Kolkata) approved the study protocol (IRB No: ERC/373/Inst/WB/2013/RR-19). Written informed consent was obtained from all patients.
RESULTS
Patient characteristics
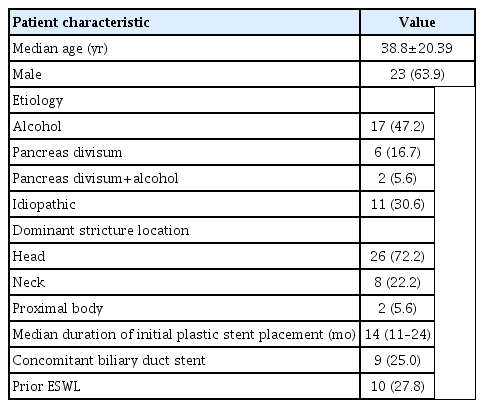
Thirty-nine patients were screened from June 2017 to May 2019 for inclusion in the study, and three patients were excluded for pancreatic pseudocyst (n=1), severe acute exacerbation of chronic pancreatitis (n=1), and refusal to participate (n=1). The mean age (±standard deviation) of the 36 patients involved in the study was 38.8±20.39 years; 23 (63.9%) of them were male. The etiology of CP was chronic alcohol abuse in 17 patients (47.2%), pancreas divisum in 6 (16.7%), alcohol abuse plus pancreas divisum in 2 (5.6%), and idiopathic in 11 (30.6%). The median duration from initial PS placement was 14 months (IQR, 11–24 months). The median number of previous ERP procedures was 2.8 (IQR, 2–5). Nine patients (25.0%) had biliary strictures secondary to chronic pancreatitis and required concomitant biliary duct stenting. Ten patients (27.8%) had large stones (>5 mm) in the MPD and underwent ESWL before FCSEMS placement. Table 1 summarizes the baseline characteristics of all patients with refractory MPD strictures. All patients had a previous pancreatic sphincterotomy prior to FCSEMS placement. FCSEMSs were successfully placed in all patients (100% technical success). The FCSEMSs used were 6 mm or 8 mm in diameter and 8 cm in length. The FCSEMSs were inserted through the major papilla in 28 (77.8%) patients, and through the minor papilla in the remaining eight (22.2%). Complete runoff of contrast material with a pancreatolith or protein plug occurred after immediate stent deployment in all patients. Figure 1 shows a tight pancreatic duct stricture in which balloon dilatation was performed, and a Niti-S [Bumpy] stent was placed.

(A) Pancreatogram obtained before placement of the metal stent showing a tight stricture in the head of the pancreatic duct. (B) Dilatation of pancreatic duct stricture by the hydrostatic balloon. (C) Pancreatogram after Niti-S [Bumpy] stent deployment. (D) Follow-up pancreatogram at 3 months showing resolution of the pancreatic duct stricture.
Clinical outcomes
Clinical success was achieved in 31 of 36 patients (86.1%). The median VAS pain score before stenting was 7.5 (range, 5.25–9), while that 4 weeks post-procedure was 2 (range, 1–6.5), with a statistically significant improvement (p<0.001). All FCSEMSs (except for migrated cells) were easily removed using snare forceps. Distal migration of the FCSEMS was noted in six (16.7%) patients. Five patients experienced asymptomatic migration with stricture resolution detected at a median 148 days; hence, no additional stenting was performed. FCSEMS migration was diagnosed in 1 patient because of pancreatitis 36 days after stent placement. The patient had a persistent MPD stricture, and two pancreatic PSs exchanges were performed. No proximal migration of the FCSEMS was observed. The median indwelling stent period was 156 days (IQR, 65–186 days). An inflated retrieval balloon (Telmed Systems, Hudson, MA, USA) easily passed through the pancreatic duct strictures in 29 (80.6%) patients. The median diameter of the stricture significantly increased from 1.7 mm (IQR, 1.1–2.4 mm) to 3.5 mm (IQR, 2.6–4.8 mm) after FCSEMS deployment (p<0.001).
Long-term outcomes were evaluated in 18 patients who underwent follow-up for a median of 19 months (IQR, 8–23 months). Ten (55.6%) patients remained asymptomatic. In 8 (44.4%) symptomatic patients, repeat FCSEMS placement (n=2), PS placement (n=4), or surgery (n=2) was performed. The clinical outcomes of all patients are summarized in Table 2.
Adverse events
Early complications after FCSEMS placement were mild abdominal pain in 8 (22.2%) patients controlled with analgesics. Three patients developed intolerable abdominal pain with normal amylase and lipase levels, and mild pancreatitis in one patient. Despite adequate analgesics, early stent removal within a week was done in two patients due to intolerable pain. One week after FCSEMS, the patient was readmitted because of worsening pain and underwent endosonography-guided celiac plexus block. Three (8.3%) patients developed stent-induced de novo ductal strictures (two had received 8-mm-diameter FCSEMSs and one had received a 6-mm-diameter FCSEMS) that were incidentally diagnosed at the time of stent removal. All three patients received a 3-month pancreatic PS across the stricture. These strictures were completely resolved at the 3-month follow-up. Figure 2 shows the occurrence of a de novo pancreatic duct stricture after stent removal, for which a plastic pancreatic duct stent was placed across the stricture. No pancreatic sepsis was observed during stent placement. Adverse events related to FCSEMS placement in all patients are summarized in Table 3.

(A) Occurrence of a de novo pancreatic duct stricture after stent removal. (B) Placement of a plastic pancreatic duct stent across the stricture.
DISCUSSION
PS placement exchange every 6 months for at least a year is the standard of care for the endoscopic treatment of dominant, painful MPD strictures. The technical success rate of deploying a PS across the dominant MPD stricture is reported to be close to 90%.19 In a meta-analysis of nine studies, long-term pain relief was reported in 67.5% of 536 patients.20 MPD strictures that persist or relapse beyond 1 year after single pancreatic stent placement are defined as refractory strictures. These strictures are challenging to treat, and the present management options include multiple side-by-side PSs, FCSEMSs, or surgery. Surgical treatment is a definitive option with optimal results.21 However, surgery is not readily accepted by patients due to the perception of its invasiveness, and some patients with CP are not eligible because of their comorbidities. In patients with tight strictures, placement of multiple PSs is technically challenging, and these stents require frequent exchanges. Placement of FCSEMSs is relatively easier as they have greater flexibility, lesser pushing catheter external diameter (8.5 F vs. 10 F), and longer stent patency rates, since the larger diameter exerts a radial expansion force; subsequently, cost is reduced due to the need for fewer endoscopic procedures.17 Therefore, we prefer FCSEMSs over multiple PSs as a treatment option for refractory MPD strictures due to CP.
The present study is possibly the largest report of FCSEMSs in the treatment of refractory MPD strictures. Our study demonstrated 100% technical success and 86.1% clinical success with a statistically significant increase in MPD stricture size. These results are similar to those of previously published reports14-18,22-24 except for the stricture resolution rate, which was close to 40% in two reports.17,22
In the present study, we used Niti-S [Bumpy] FCSEMS, which has flared ends that act as anti-migration features and a varying cell size throughout the stent to obtain differential radial forces. Silicone is used in the covering membrane at the ends of the stent to create the flare. The middle portion of the cover is made of polytetrafluoroethylene for high conformability. The stent introducer was 8.5F. The Bumpy stent is available with a diameter of 6, 8, or 10 mm and varying lengths (4–12 cm). The stent has a lasso at the proximal end for easy removal. The FCSEMS indwelling period had a range of 2 to 3 months in previous reports to prevent the development of ductal strictures.15-17,22 However, in the present study, the median FCSEMS indwelling period was 156 days, with only 8.3% of patients developing de novo strictures, which were asymptomatic and resolved after 3 months of single PS placement.
As shown in Table 4, we compared various studies using the Niti-S [Bumpy] and new non-flared FCSEMSs.14,16-18 The spontaneous stent migration rate of 16.7% in the present study is similar to that (14.1%) reported in a recently conducted meta-analysis of 163 patients.25 In the study by Trignali et al., the FCSEMS length was 3 cm with the supposed advantages of reducing the side branch occlusion and exactly covering the MPD stricture to prevent damage. However, the 3-cm stent had a very high migration rate of 46%, with 27% of patients developing FCSEMS-induced stricture, a rate that is higher than that in the present study.14 Thus, we recommend using a longer FCSEMS with a 6-month indwelling period for the best results.

Clinical outcomes of various studies after placement of Niti-S [Bumpy] stent and novel non-flared FCSEMS
One patient in our study developed severe pain not controlled with analgesics following FCSEMS placement, which was eventually treated with celiac plexus block. This implies that pain following FCSEMS placement is majorly due to duct dilatation and is temporary, as pain relief was sustained until the 6-month follow-up after stent removal. However, further studies are required to confirm this assumption.
The long-term resolution of MPD stricture after FCSEMS application is essential for decreasing repeated treatments and improving patients’ quality of life. In the present study, long-term data over a median 19 months showed that 55.6% of patients remained asymptomatic after FCSEMS removal, while the rest of the patients required different treatment modalities for pain recurrence. In two previous studies of the same FCSEMS type, pain relief was sustained for 89% and 37% of patients over a median follow-up of 39 and 35 months, respectively.14,17 The heterogeneity in these results cannot be explained, and controlled comparative trials are needed to understand its natural history.
This study has some limitations. This was a single-center prospective study that did not include a control group or a comparison with multiple PSs. Long-term outcomes were evaluated in only 50% of the patients. Further comparative or randomized control studies with greater patient numbers are necessary to confirm the efficacy, feasibility, and safety of FCSEMSs for the treatment of refractory MPD strictures. However, in the present study, FCSEMS placement showed good technical and clinical success rates and achieved pain relief in patients with refractory MPD strictures.
Notes
Conflicts of Interest
The authors have no potential conflicts of interest.
Funding
None.
Author Contributions
Conceptualization: BBS, MKG; Data curation: BBS, MKG; Formal analysis: BBS, GAR, UG, SA, MKG; Investigation: UG; Project administration: BBS, MKG; Resources: BBS, MKG; Software: BBS; Validation: MKG; Writing–original draft: BBS; Writing–review & editing: BBS, GAR, UG, SA, MKG.