MÃĐnÃĐtrier disease (MD) is a rare, acquired gastric hyperproliferative disorder characterized by enlarged gastric folds, hypoalbuminemia, and reduced acid production.1 Histological hallmarks include a massive expansion of surface mucous cells (foveolar hyperplasia) and glandular atrophy.2 Adults and children have different etiologies, clinical manifestations, and outcomes. It is a chronic condition in adulthood with a risk of neoplastic transformation of up to 9% after 10 years of diagnosis.3 During childhood, it is considered an acute, self-limiting condition, with an average disease duration of 2 weeks.4 Pathogenesis is still unclear, which might limit the management of patients suffering from this condition.
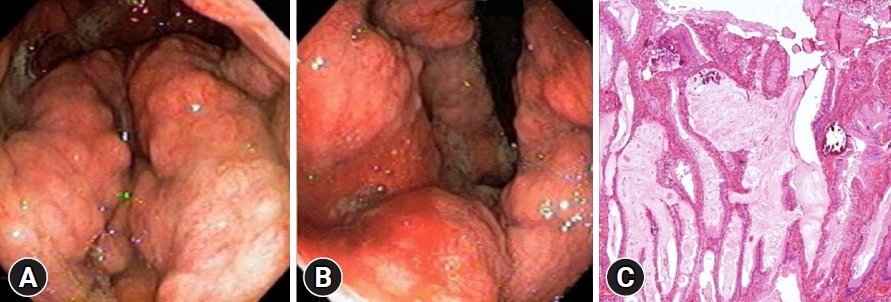
This article reports the first case in the literature of chronic pediatric MD leading to gastric cancer. A previously healthy 10-year-old girl presented with vomiting, sporadic melena, and weight loss (10% of body weight) over one month. A physical examination revealed pallor and mild edema. Laboratory studies demonstrated anemia (hemoglobin [Hb], 5.0 g/dL), hypoalbuminemia (3.4 mg/dL), and an elevated serum gastrin level (350 pg/mL). Esophagogastroduodenoscopy (EGD) revealed decreased gastric distensibility and hypertrophic gastric folds in the corpus and fundus with thick mucus, sparing the antrum (Figs. 1A, B). Gastric biopsies were inconclusive; therefore, repeat EGD with deep snare biopsy was performed. The histopathological assessment revealed massive foveolar hyperplasia, tortuous gland ducts with cystic dilatation, and atrophy of chief and parietal cells, confirming MD (Fig. 1C). Immunohistochemistry testing for cytomegalovirus (CMV) and histopathological and urease tests for Helicobacter pylori demonstrated negative results.
The patient was managed with supportive treatment, oral iron supplementation, and a high-protein diet. She presented with slow progressive improvement after 4 months with weight gain and resolution of vomiting, and normalization of Hb and serum albumin levels. However, the endoscopic findings were not resolved.
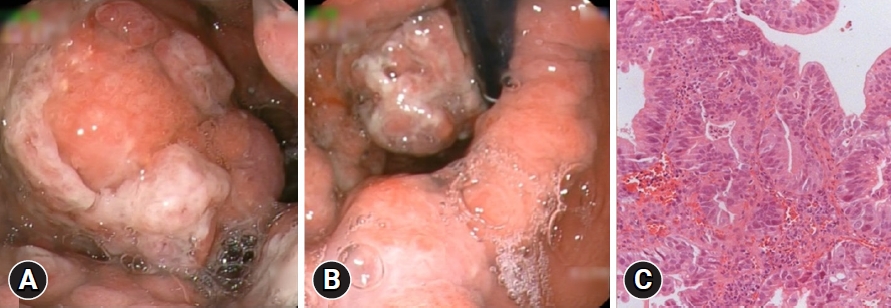
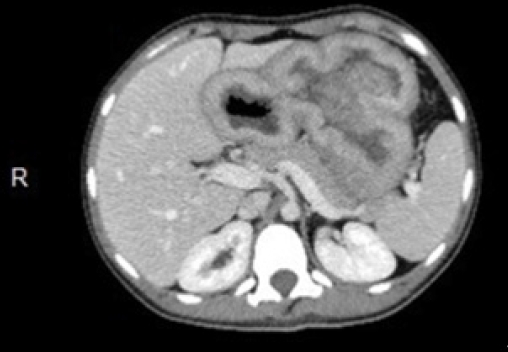
She maintained close clinical, laboratory, and endoscopic follow-up. After 8 years, biochemical studies revealed hypoalbuminemia (3.0 mg/dL) and mild anemia (Hb 11.8 g/dL). EGD demonstrated a protruding lesion (Paris Classification type 0âIs) located at the greater curvature of the corpus with friability, loss of glandular pattern, and altered vascular pattern (Figs. 2A, B). Biopsies confirmed the presence of moderately differentiated tubular adenocarcinomas (Fig. 2C). A computed tomography scan showed a large mass protruding from the greater curvature measuring 7.2Ã5.5Ã5.0 cm and diffuse parietal thickening of the gastric background mucosa with no signs of metastatic disease (Fig. 3).
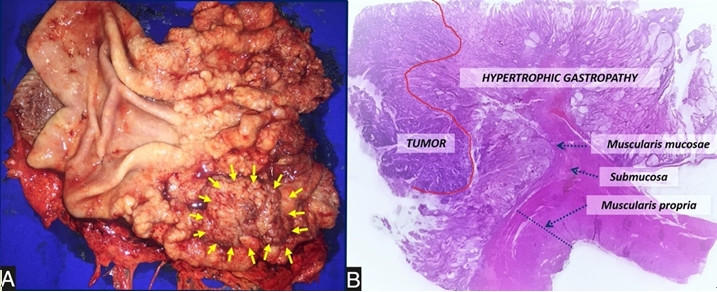
The patient underwent laparoscopic total gastrectomy with D2 lymphadenectomy (Fig. 4A). Histopathological analysis confirmed an intramucosal adenocarcinoma (T1a) with free margins and no lymphovascular invasion (Fig. 4B). The postoperative course was successful without any complications, and the patient was discharged on the 14th postoperative day with well-tolerated oral feeding. Adjuvant therapy was not required, and there was no recurrence of the disease after eight years of follow-up.
MD is considered a self-limiting disease in children. There are approximately 150 reported cases of pediatric MD, and the only prolonged case was described by Faure et al.,5 in which a 7-year-old girl with MD maintained endoscopic gastric alterations at her 26-month follow-up. However, this patient was asymptomatic with a progressive histological improvement of the gastric lesions. To our knowledge, this is the first case in the literature of chronic MD in a child with gastric cancer.
The present case modifies the clinical rationale for treatment of a case of MD in a young patient. As opposed to previous findings,3,4 we believe that endoscopic follow-up is indicated until normalization of the gastric alterations since the disease may become chronic. If the disease does not resolve, the patient should undergo close gastric cancer screening. Depending on the clinical symptoms and laboratory studies, total gastrectomy might be considered, similar to the management of adult MD.
Interestingly, the reported case did not present any known triggers associated with MD. CMV is the most commonly described agent in children, with a prevalence of approximately 60% among patients with pediatric MD.4 Furthermore, CMV infection has also been reported in adults.6,7 H. pylori is another frequently associated agent.8 However, as both CMV and H. pylori can often be detected in asymptomatic patients, it is difficult to confirm the cause-and-effect relationship. Immune-mediated disorders, such as ulcerative colitis and sclerosing cholangitis, have also been reported in patients with MD, suggesting an immune component in its pathogenesis.1 Genetic predisposition probably plays a role in the development of the disease.9,10
The etiology and pathogenesis of MD have not yet been fully elucidated. Current knowledge suggests that triggers induce local overproduction of transforming growth factor-alpha in the stomach, leading to increased tyrosine kinase epidermal growth factor receptor signaling.1 It results in a shift in the gastric progenitor cell differentiation, leading to a marked increase in mucosal surface cells and consequent reduction in parietal and chief cells.3 Gastrin levels remain normal to moderately elevated despite the hypochlorhydria.2 Increased mucin synthesis and the widening of tight junctions result in gastric protein loss.
Children usually present with an abrupt onset of nausea, vomiting, abdominal pain, anorexia, weight loss, diarrhea, and edema,8 whereas adults usually present with insidious onset. Diagnosis is suggested by EGD findings, such as giant rugal folds in the corpus and fundus, with relative sparing of the antrum, occasionally with erosions, decreased distensibility, and a large amount of thick mucus. Routine biopsies may not confirm the disease because superficial samples assess only the foveolar compartment. Therefore, total mucosal samples with deep snare biopsies are necessary to evaluate mucosal architecture and pit-to-gland ratio.6
In conclusion, this case report raises concerns regarding the management of MD in children and may change the clinical rationale for pediatric patients with such a disease. We suggest endoscopic surveillance until normalization of gastric changes, even if clinical and laboratory remission is achieved. Additionally, gastrectomy should be considered in severe chronic cases, similar to that in the adult population. Written informed consent was obtained from all patients.