AbstractBackground/AimsCapsule enteroscopy (CE) and intestinal ultrasonography (IUS) are techniques that are currently used for investigating small-bowel (SB) diseases. The aim of this study was to compare the main imaging findings and the lesion detection rate (LDR) of CE and IUS in different clinical scenarios involving the SB.
MethodsWe retrospectively enrolled patients who underwent CE and IUS for obscure gastrointestinal bleeding (OGIB), complicated celiac disease (CeD), and suspected or known inflammatory bowel disease (IBD). We evaluated the LDR of both techniques. The accuracy of IUS was determined using CE as the reference standard.
ResultsA total of 159 patients (113 female; mean age, 49┬▒19 years) were enrolled. The LDR was 55% and 33% for CE and IUS (p<0.05), respectively. Subgroup analysis showed that the LDR of CE was significantly higher than that of IUS in patients with OGIB (62% vs. 14%, p<0.05) and CeD (55% vs. 35%, p<0.05). IUS showed a similar LDR to CE in patients with suspected or known IBD (51% vs. 46%, p=0.83).
INTRODUCTIONFor a long time, the small bowel (SB) was considered a diagnostic ŌĆ£black boxŌĆØ1 because of the difficulties in exploring this organ, both endoscopically and radiologically. However, in the last decade, novel endoscopic devices2 and imaging techniques3 have been developed. Among them, capsule enteroscopy (CE) is now considered the reference standard in the diagnosis of SB diseases,4 although it is expensive and not widely available in all medical centers. Conversely, intestinal ultrasonography (IUS) is a ŌĆ£low-costŌĆØ and easily repeatable technique that is largely available in European countries.5
In the assessment of SB diseases, IUS is currently used in patients who are clinically suspected of having appendicitis6 and inflammatory bowel disease (IBD).7,8 In this context, the typical ultrasonographic signs are thickening of the bowel wall,9 increased vascularization within the bowel wall, dilatation of the SB loops, enlargement of the mesenteric lymph nodes, hypertrophy of the mesentery, and presence of free abdominal fluid within the bowel loops.10 In the diagnosis of CrohnŌĆÖs disease, IUS shows a similar accuracy to magnetic resonance imaging or computed tomography,11 especially when the disease is localized in the terminal ileum, whereas lower diagnostic accuracy rates are reported for more proximal disease localizations. Furthermore, in these patients, IUS allows the detection of extraluminal complications such as abscesses and fistulas.11,12 IUS also plays a role in ruling out malabsorption syndromes and in the diagnosis and follow-up of celiac disease (CeD), the most common ultrasonographic findings of which are bowel dilatation, increased peristalsis, lymph node enlargement, and free abdominal fluid.12,13
According to international guidelines, CE plays a pivotal role in the diagnosis and management of SB disorders.4 Obscure gastrointestinal bleeding (OGIB) is the most relevant indication of CE; however, CE is also currently used for the evaluation and monitoring of CrohnŌĆÖs disease.8 CE is also indicated for the surveillance of familial polyposis syndromes, suspected SB tumors, and selected cases of CeD.14 In a recent meta-analysis, CE showed the highest lesion detection rate (LDR) among all other diagnostic procedures in the assessment of OGIB and CrohnŌĆÖs disease.15 Furthermore, CE has been demonstrated to be better than optical endoscopy in predicting villous atrophy, owing to its greater concordance with histology.14
Despite the widespread use of CE and IUS especially in IBD centers, only a few studies have compared these two modalities. Aloi et al. showed that oral contrast ultrasonography of the SB is more effective in the diagnosis of terminal ileitis, whereas CE has a higher LDR in the proximal and middle ileum16; however, these two techniques present a similar sensitivity in the jejunum, without demonstrating statistically significant differences in overall performance. A meta-analysis by Kopylov et al.17 showed that SB contrast ultrasonography and CE presented a similar LDR in active CrohnŌĆÖs disease, with CE showing superior accuracy in detecting proximal lesions. A similar LDR was also reported by Carter et al.,18 in a study in which CE was used as the reference standard in a population with suspected CD after negative ileo-colonoscopy, suggesting that ultrasonography can successfully demonstrate active inflammation similar to CE or other cross-sectional imaging modalities. Nevertheless, no study has compared CE and SB ultrasonography in routine clinical practice.
The aim of this study was to compare the main imaging findings and the LDR of CE and IUS in different clinical scenarios involving the SB.
METHODSPatientsWe retrospectively evaluated consecutive patients referred to Fondazione IRCCS CaŌĆÖ Granda Ospedale Maggiore Policlinico (Milan, Italy) who underwent both CE and IUS between January 2011 and May 2018. Patients who underwent CE and IUS within a 6-month period for a known or suspected SB disorder were included.
As a data acquisition strategy, we first evaluated patients who underwent CE, which was considered the limiting factor. Among them, we subsequently analyzed those who underwent an IUS within the established time frame (6 months).
For each enrolled patient, the following data were collected: demographic data, indication of CE/IUS and corresponding technical aspects (see the following paragraphs), body mass index, comorbidities, final diagnosis, and LDR (defined as the proportion of positive tests with findings compatible with the clinical suspicion).
Capsule enteroscopyBefore undergoing CE (PillCam SB; Given Imaging, Yoqneam, Israel), the patients were prescribed a standard bowel preparation consisting of a low-fiber diet during the 3 days before the examination, a clear liquid diet, and ingestion of 2 L polyethylene glycol on the day before the examination. The recorder was placed following the manufacturerŌĆÖs suggestions, and the acquired data were analyzed using a dedicated software (given imaging) by an expert reader who performs >100 CE investigations per year. Data acquisition lasted until capsule battery depletion.
The following parameters were assessed: CE completeness, bowel preparation quality, transit time, and endoscopic findings. Cases of incomplete CE (i.e., the cecum was not reached) were excluded from the analysis.
Intestinal ultrasonographyAfter an overnight fast, the patients underwent IUS performed by experienced and dedicated ultrasonographers. Philips iU22 (Philips Ultrasound; Philips Healthcare, Bothell, WA, USA) with a multifrequency convex probe (C5-2, 5ŌĆō2 MHz) and a linear probe (L12-5, 12ŌĆō5 MHz) was used.
The following parameters were evaluated: SB wall thickness (parietal thickness >0.3 cm was considered pathological),5 SB dilatation (transverse diameter >2.5 cm),10 mesenteric lymph node enlargement (>1 cm longitudinal axis and >0.5 cm short axis), mesenteric hypertrophy, and free abdominal fluid >150 mL within the bowel loops. The IUS result was considered pathological when at least one of the above-mentioned parameters was present.
Statistical analysisAll statistical analyses were performed using GraphPad Prism (release 6.0; GraphPad Software Inc., La Jolla, CA, USA). Continuous data are presented as mean (┬▒standard deviation) with the corresponding 95% confidence interval (CI). Categorical variables were compared using the chi-square or Fisher exact test. Statistical significance was set at p<0.05. We calculated the sensitivity, specificity, positive and negative predictive values, and positive and negative likelihood ratios of IUS, with CE as the reference standard. The agreement between the two techniques was calculated using the ╬║ coefficient and its 95% CI. The agreement was judged as poor (╬║, 0ŌĆō0.20), fair (╬║, 0.21ŌĆō0.40), moderate (╬║, 0.41ŌĆō0.60), substantial (╬║, 0.61ŌĆō0.80), or almost perfect (╬║, 0.81ŌĆō1.00).
Ethical statementsThis study was approved by the local ethics committee (Comitato Etico Milano Area 2, protocol no. 137/2021), and the requirement for informed consent was waived owing to the retrospective study design. This study was conducted in compliance with the Declaration of Helsinki (1964) and all its subsequent amendments. The data were collected within the framework of standard patient care. Patients were treated confidentially, in compliance with the most recent privacy laws at the European and national levels, and were anonymized. Therefore, the investigators who analyzed the data were blinded to the patientsŌĆÖ identities.
RESULTSPatientsWe retrospectively enrolled 159 consecutive patients who were referred to Fondazione IRCCS CaŌĆÖ Granda Ospedale Maggiore Policlinico in Milan and underwent CE and IUS. The indications were OGIB in 35 patients (22%), CeD in 81 patients (51%), and suspected or known IBD in 43 patients (27%). The clinical and demographic characteristics of the patients are reported in Table 1.
CE and IUSIn two cases (1%), the capsule did not reach the cecum because of slow intestinal transit. Therefore, 157 CE cases were analyzed. The capsule was retained in two cases (1%) owing to the presence of stenosis. IUS was successfully performed in all cases, and the patientsŌĆÖ body constitutions and the presence of intestinal meteorism did not interfere with the examination. The technical aspects of the CE and IUS investigations are reported in Table 2.
Overall, the LDR of IUS and CE for SB alterations was 33% (95% CI, 25%ŌĆō40%) and 55% (95% CI, 46%ŌĆō62%), respectively (p<0.001). The sensitivity, specificity, positive and negative predictive values, and positive and negative likelihood ratios of IUS for the identification of any diseases or endoscopic signs observed during CE are reported in Table 3. The LDRs of CE and IUS are reported in Table 3.
Figure 1 shows the corresponding CE finding (in percentage) for every ultrasonographic parameter.
Forty-seven patients (30%) had a negative IUS and a positive CE. In these cases, CE detected lesions mostly in the proximal part of the SB (72%; duodenum, 23%). Multiple and extensive lesions were observed in 25%, whereas the lesions were localized in the ileum in 8% of the cases.
In 13 patients (8.1%), ultrasonographic examinations revealed relevant findings not detected by CE. Among these patients, 77% had SB loop dilatation and 54% had a diagnosis of CeD or nonceliac intestinal atrophy.
Obscure gastrointestinal bleedingThirty-four patients (21%) were investigated for OGIB (including one patient in whom the capsule did not reach the cecum). The LDR was 14% (95% CI, 4%ŌĆō30%) and 62% (95% CI, 43%ŌĆō78%) for IUS and CE, respectively (p<0.001). The IUS findings in patients with OGIB are reported in Table 2. The IUS findings with the corresponding CE findings are shown in Figure 1. In the OGIB group, the most frequent ultrasonographic finding was free abdominal fluid in five patients (14%) and increased SB wall thickness in four patients (11%), which corresponded to endoscopic findings of nonspecific signs of inflammation in three patients.
Celiac diseaseEighty-one patients (51%) with known CeD were investigated (one patient was excluded from the analysis because the capsule did not reach the cecum owing to slow bowel transit). Of these patients, 72 (45%) had a suspected complicated CeD and eight patients (5%) had a known complicated CeD. The LDR was 35% (95% CI, 24%ŌĆō46%) and 55% (95% CI, 43%ŌĆō66%) for IUS and CE, respectively (p=0.011). The IUS findings in patients with CeD are reported in Table 2. The IUS findings with the corresponding CE findings are shown in Figure 1.
In this subgroup, the most commonly observed ultrasonographic finding was SB loop dilatation (15 cases, 18%), which was associated with endoscopic signs of mucosal atrophy in 66% of the cases. Lymph node enlargement was present in 10 cases (12%), 90% of which were associated with macroscopic signs of atrophy. Increased SB wall thickness was detected in seven cases (8%), 57% of which were associated with atrophy.
Suspected or known IBDForty-three patients (27%) were investigated for suspected or known IBD. The LDR was 46% (95% CI, 32%ŌĆō61%) and 51% (95% CI, 36%ŌĆō65%) for IUS and CE, respectively (p=0.83). The IUS findings in patients with suspected or known IBD are reported in Table 2. The IUS findings with the corresponding CE findings are shown in Figure 1.
In this subgroup, the most frequent ultrasonographic sign was free abdominal fluid (12 cases, 28%), which corresponded to mucosal erosions and nonspecific signs of inflammation in 33% of the cases. Another common ultrasonographic sign was increased SB wall thickness (11 cases, 25%) corresponding to endoscopic signs of inflammation (five cases, 45%) and erosions (seven cases, 63%). SB loop dilatation was observed in 10 cases (23%), which corresponded to different endoscopic findings (nonspecific signs of inflammation in two cases, macroscopic signs of atrophy in two cases, and angiectasia in two cases).
DISCUSSIONThe investigation and visualization of the SB have been challenging issues in daily clinical practice.18 CE and IUS were recently introduced for the diagnosis and follow-up of SB disorders; however, only a few comparative studies limited to patients with IBD have been published.17 From this point of view, the present study represents the first attempt to fill this research gap.
IUS is a noninvasive and low-cost technique that is feasible as a bedside point-of-care test or can be performed during outpatient clinic visits.11,12 These advantages can support its routine use for a rapid decision-making process. In particular, the detection of any of the most relevant IUS parameters (SB dilatation, SB wall thickening, lymph node enlargement, and free abdominal fluid) can indicate the need for an extensive investigation of the intestinal tract (i.e., CE). After the identification of pathological findings on ultrasound, the investigation can be repeated after a definite time to monitor the response to therapy and evaluate extraintestinal signs of the disease. Moreover, the recent implementation of novel ultrasound techniques that can measure tissue elasticity may further improve the diagnostic ability of IUS.19
Meanwhile, CE is more invasive and expensive than IUS and can have some relative or absolute contraindications, such as previous SB surgery with enteric anastomosis and the presence of symptoms suggesting occlusion. In addition, CE also has some possible adverse effects such as retention, which can potentially lead to surgical extraction of the retained capsule. The retention risk is approximately 1%, although it can be higher in patients with IBD.20 However, direct visualization of the SB mucosa provides an advantage in the diagnosis and follow-up of SB diseases.4
In our study, CE presented a higher LDR than IUS (55% vs. 33%). In addition, CE was confirmed to be the appropriate reference standard in the assessment of SB diseases. However, as the two techniques investigate different aspects of the same problem, IUS can have a complementary role in the evaluation of the SB, such as in cases of CeD and suspected or known enteropathy. Furthermore, the mean procedure time of IUS is 15ŌĆō20 minutes, whereas the overall procedure and interpretation time of CE is approximately 16 hours (4 hours of preparation, 10 hours of recording time, and 60 to 120 minutes of interpretation, depending on the examinerŌĆÖs expertise); thus, IUS may represent an important and rapid upfront technique for primary assessment. In the case of OGIB, the difference between the LDR of IUS and that of CE is high, making IUS useless in most cases in this clinical scenario. This is because OGIB is frequently caused by flat and small vascular lesions usually not involving the SB wall and, therefore, is not detectable with IUS. In fact, in the OGIB subgroup, the LDR of IUS was 14% (vs. 62% for CE) without a clear association between IUS signs and endoscopic markers. Thereby, we do not recommend the use of IUS in the OGIB setting.
Among the patients investigated for CeD, the LDR was 35% and 55% for IUS and CE, respectively. The most frequently observed endoscopic finding in these cases was atrophy, which was more common in patients with complicated CeD than in those with uncomplicated disease (75% vs. 43%, unpublished data). Meanwhile, the more frequently identified IUS signs were SB loop dilatation and lymph node enlargement, which are reported to be the most sensible and specific findings for the diagnosis of CeD.13 As mentioned above, there was a high rate of correspondence between these findings and the presence of macroscopic signs of atrophy on CE. As patients with CeD have an increased risk of intestinal neoplasms, parietal assessment is necessary. The identification of increased thickness, stenosis, or dilatation, as well as the visualization of pathological lymph nodes can support the search for endoscopic malignancies.
Among the patients investigated for known or suspected IBD, the two techniques showed a similar LDR (46% vs. 51%, p>0.05). In this setting, as previously reported,11,12 the most frequent ultrasonographic sign identified was increased SB wall thickness, which corresponded to endoscopic signs of inflammation and mucosal erosions, both of which are specific for IBD.
Overall, our findings suggest that increased SB wall thickness indicates an inflammatory state in 80% of the cases; SB dilatation can suggest the presence of atrophy with or without mild nonspecific inflammation in 50% of the cases; lymph node enlargement is a sign associated with both atrophy and inflammation; mesenteric hypertrophy is usually a rare finding presenting in cases of severe inflammation; and free abdominal fluid is detected in cases of mild to severe inflammation.
The present study is the first to compare CE and IUS in this clinical scenario. However, several limitations should be underlined. This was a retrospective study and, therefore, patients were not randomly selected. As IUS is not considered in the diagnostic workup of OGIB, only 6% of patients who underwent CE examination in this subgroup also underwent IUS. Because 89% of the IUS procedures were performed before CE, blinding was possible only in ultrasonography cases.
In conclusion and considering the above-mentioned limitations, our study confirmed that CE is diagnostically superior to IUS, especially when the disease is localized in the more proximal parts of the SB. Despite its limitations, IUS can play a role as a complementary tool and a rapid bedside test. In more challenging cases, the two techniques can be sequentially used to increase the probability of reaching a final diagnosis. Further studies are needed to assess whether adding oral contrast to the baseline examination can further improve the sensitivity of IUS in selected cases.
NOTESFig.┬Ā1.Correspondence between ultrasonographic signs and findings of capsule enteroscopy divided into atrophy, erosive inflammation, and nonerosive inflammation. The percentages in black indicate the correspondence with atrophy, those in red indicate the correspondence with erosive inflammation, and those in orange indicate the correspondence with nonerosive inflammation. 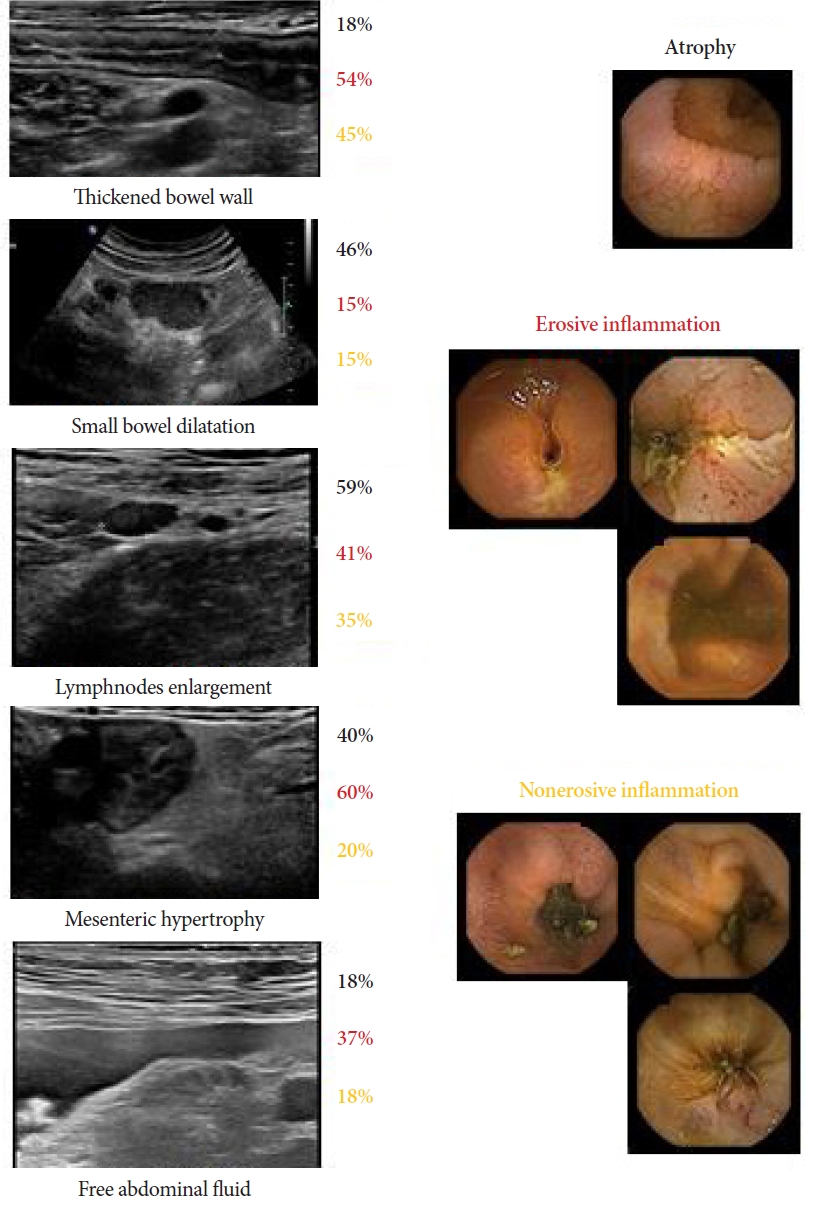
Table┬Ā1.Clinical and demographic characteristics of patients included in the study Table┬Ā2.Technical aspects of capsule enteroscopies and ultrasonographies performed
Table┬Ā3.Diagnostic accuracy of small-bowel ultrasonography vs. capsule enteroscopy as reference standard REFERENCES1. Monkemuller K. Should we illuminate the black box of the small bowel mucosa from above or below? Clin Gastroenterol Hepatol 2012;10:917ŌĆō919.
3. Fidler JL, Goenka AH, Fleming CJ, et al. Small bowel imaging: computed tomography enterography, magnetic resonance enterography, angiography, and nuclear medicine. Gastrointest Endosc Clin N Am 2017;27:133ŌĆō152.
4. Pennazio M, Spada C, Eliakim R, et al. Small-bowel capsule endoscopy and device-assisted enteroscopy for diagnosis and treatment of small-bowel disorders: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline. Endoscopy 2015;47:352ŌĆō376.
5. Maconi G, Bianchi Porro G. Ultrasound of the gastrointestinal tract. 2nd ed. Berlin, Heidelberg: Springer; 2014.
6. Wale A, Pilcher J. Current role of ultrasound in small bowel imaging. Semin Ultrasound CT MR 2016;37:301ŌĆō312.
7. Parente F, Maconi G, Bollani S, et al. Bowel ultrasound in assessment of CrohnŌĆÖs disease and detection of related small bowel strictures: a prospective comparative study versus x ray and intraoperative findings. Gut 2002;50:490ŌĆō495.
8. Fraquelli M, Colli A, Casazza G, et al. Role of US in detection of Crohn disease: meta-analysis. Radiology 2005;236:95ŌĆō101.
9. Nylund K, Hausken T, Odegaard S, et al. Gastrointestinal wall thickness measured with transabdominal ultrasonography and its relationship to demographic factors in healthy subjects. Ultraschall Med 2012;33:E225ŌĆōE232.
10. Nylund K, Maconi G, Hollerweger A, et al. EFSUMB recommendations and guidelines for gastrointestinal ultrasound: part 1: examination techniques and normal findings (long version). Ultraschall Med 2017;38:e1ŌĆō15.
11. Fraquelli M, Castiglione F, Calabrese E, et al. Impact of intestinal ultrasound on the management of patients with inflammatory bowel disease: how to apply scientific evidence to clinical practice. Dig Liver Dis 2020;52:9ŌĆō18.
12. Nylund K, Odegaard S, Hausken T, et al. Sonography of the small intestine. World J Gastroenterol 2009;15:1319ŌĆō1330.
13. Fraquelli M, Colli A, Colucci A, et al. Accuracy of ultrasonography in predicting celiac disease. Arch Intern Med 2004;164:169ŌĆō174.
14. Branchi F, Locatelli M, Tomba C, et al. Enteroscopy and radiology for the management of celiac disease complications: time for a pragmatic roadmap. Dig Liver Dis 2016;48:578ŌĆō586.
15. Marmo R, Rotondano G, Piscopo R, et al. Meta-analysis: capsule enteroscopy vs. conventional modalities in diagnosis of small bowel diseases. Aliment Pharmacol Ther 2005;22:595ŌĆō604.
16. Aloi M, Di Nardo G, Romano G, et al. Magnetic resonance enterography, small-intestine contrast US, and capsule endoscopy to evaluate the small bowel in pediatric CrohnŌĆÖs disease: a prospective, blinded, comparison study. Gastrointest Endosc 2015;81:420ŌĆō427.
17. Kopylov U, Yung DE, Engel T, et al. Diagnostic yield of capsule endoscopy versus magnetic resonance enterography and small bowel contrast ultrasound in the evaluation of small bowel CrohnŌĆÖs disease: systematic review and meta-analysis. Dig Liver Dis 2017;49:854ŌĆō863.
18. Carter D, Katz LH, Bardan E, et al. The accuracy of intestinal ultrasound compared with small bowel capsule endoscopy in assessment of suspected CrohnŌĆÖs disease in patients with negative ileocolonoscopy. Therap Adv Gastroenterol 2018;11:1756284818765908.
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
