Comparison of diagnostic performances of slow-pull suction and standard suction in endoscopic ultrasound-guided fine needle biopsy for gastrointestinal subepithelial tumors
Article information
Abstract
Background/Aims
Endoscopic ultrasound-guided fine-needle biopsy (EUS-FNB) is integral to the diagnosis of gastrointestinal (GI) subepithelial tumors (SETs). The impact of different EUS-FNB tissue sampling techniques on specimen adequacy and diagnostic accuracy in SETs has not been fully evaluated. This study aimed to compare the diagnostic outcomes of slow-pull (SP) and standard suction (SS) in patients with GI SETs.
Methods
In this retrospective comparative study, 54 patients were enrolled. Medical records were reviewed for location and size of the target lesion, FNB needle type/size, technical order, specimen adequacy, diagnostic yield, and adverse events. The acquisition rate of adequate specimens and diagnostic accuracy were compared according to EUS-FNB techniques.
Results
The mean lesion size was 42.6±36.4 mm, and most patients were diagnosed with GI stromal tumor (75.9%). The overall diagnostic accuracies of the SP and SS techniques were 83.3% and 81.5%, respectively (p=0.800). The rates of obtaining adequate core tissue were 79.6% and 75.9%, respectively (p=0.799). No significant clinical factors affected the rate of obtaining adequate core tissue, including lesion location and size, FNB needle size, and final diagnosis.
Conclusions
SP and SS had comparable diagnostic accuracies and adequate core tissue acquisition for GI SETs via EUS-FNB.
INTRODUCTION
Gastrointestinal (GI) subepithelial tumors (SETs) are encountered on routine endoscopy with an incidence of 0.8%–2.0%.1 Most GI SETs are benign, such as leiomyoma, schwannoma, lipoma, ectopic pancreas, and duplication cyst. GI stromal tumors (GISTs) constitute a major proportion of premalignant GI SETs2; therefore, differential diagnosis of these lesions is critical.
Endoscopic ultrasound (EUS) information is essential for evaluating GI SETs and guiding its management; however, it has limited accuracy in discriminating premalignant lesions from SETs.3,4 Tissue sampling (TS) procedures such as EUS-guided fine-needle aspiration (EUS-FNA), EUS-guided Trucut biopsy, and EUS-guided fine-needle biopsy (EUS-FNB) have been introduced to overcome this limitation.5-8 Although EUS-FNA is the basic procedure for the cytopathological diagnosis of SETs, it has suboptimal accuracy, with a rate of 34%–82%.7,9-11 Furthermore, it has limitations in terms of obtaining core tissue. EUS-Trucut biopsy solves these problems; however, it has a high rate of technical failure.5,12,13 Recently, a large multicenter study reported the superior accuracy and reduced role of rapid on-site evaluation (ROSE) in EUS-FNB compared with EUS-FNA.14 Moreover, a meta-analysis documented the different rates of adequate TS for GI SETs between EUS-FNA (80.6%) and EUS-FNB (94.9%).15 Therefore, EUS-FNB is the standard EUS-TS procedure for the diagnosis of GI SETs.
Various factors have been investigated for the effect of EUS-TS procedures on diagnostic accuracy: FNB needle type/size, number of needle passes, application of special maneuvers (fanning), endosonographer’s experience, and availability of ROSE.10,16,17 In addition, different EUS-TS techniques are important for diagnostic accuracy. Two representative techniques are available: slow-pull (SP) and standard suction (SS). SS is performed using a 10-mL syringe attached to the proximal end of the needle to provide continuous negative pressure within the target lesion during TS. Although SS is effective for obtaining an adequate amount of tissue, its use in EUS-TS may cause histological damage and increase blood contamination.18,19 In contrast, the SP technique decreases the negative pressure by withdrawing the stylet slowly from the needle after it enters the lesion. This may improve tissue adequacy and ameliorate blood contamination.20,21 However, the exact role of suction in TS remains unclear and most reports have investigated pancreatic lesions alone.22,23 Limited studies have compared SP and SS for EUS-FNB in GI SETs. Therefore, we compared the diagnostic outcomes of the EUS-TS techniques done in patients with upper GI SETs who underwent EUS-FNB.
METHODS
Eligible patients
This retrospective comparative analysis was conducted in a tertiary referral center in Korea from January 2016 to February 2020. The inclusion criteria were as follows: (1) patients with upper GI SETs confirmed by endoscopy or imaging, (2) lesions suspected to have originated from the GI wall on EUS, and (3) patients who underwent EUS-TS with SP or SS during the first two passes. The exclusion criteria were as follows: (1) inconclusive final diagnosis, (2) lower GI SETs, (3) no SETs on EUS examination, and (4) SET-like carcinomas. Initially, 58 patients underwent EUS-TS, and four patients were excluded because of an inconclusive diagnosis (n=1) and rectal lesions (n=3).
A detailed flowchart of the study is presented in Figure 1. The medical records of EUS-TS procedures were reviewed for the location and size of the target lesion, FNB needle type/size, obtained specimen adequacy, diagnostic yield, and adverse events.
Endoscopic ultrasound-guided tissue sampling and histologic assessment
EUS-FNB was performed by two experienced endosonographers (CMC and YHK). Linear-array echoendoscopes (GF-UCT240 or GF-UCT260; Olympus Medical Systems, Tokyo, Japan) were used with a ProSound Alpha 10 processor (Aloka, Tokyo, Japan). Midazolam and meperidine were used for intravenous conscious sedation. After targeting the lesion using an echoendoscope, the FNB needle was advanced into the lesion. Four types of FNB needles (EZ shot, Olympus Optical, Tokyo, Japan; Acquire, Boston Scientific, Marlborough, MA, USA; ProCore, Cook Medical Inc., Bloomington, IN, USA; ClearTip, FineMedix, Daegu, Korea) were used. The FNB needle type and size were selected at the discretion of the endosonographer. After successful puncture of the target lesion, two passes were performed using the SP and SS techniques. The technical order was allocated using a random number sheet.
For the SP technique, we gradually and continuously removed the stylet with to-and-fro movements. The SS technique was performed by removing the stylet completely and applying negative pressure using a 10-mL syringe. After sample collection, the syringe was closed and the needle was removed from the scope. To-and-fro movements (between 15 and 20 times) in different areas of the lesion were performed in a fanning manner for each pass. If an adequate specimen was not acquired from the first two passes, additional needle passes were performed at the discretion of the endoscopist.
The acquired specimens were placed in 10% buffered formalin for histological examination with hematoxylin and eosin staining. When spindle cell tumors were detected, immunohistochemical (IHC) staining was performed for the differential diagnosis of SETs. All analyses were performed by experienced GI pathologists.
Definitions and study outcomes
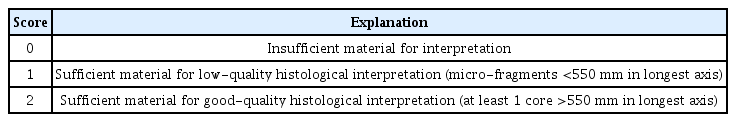
Technical success was defined as successful puncture of the target lesion. We estimated core-tissue adequacy using a previously reported scoring system.24,25 We used a 3-scale scoring system: insufficient material for interpretation (0), sufficient material with low quality for histological interpretation (1), and good quality for histological interpretation (2). Suboptimal core tissue adequacy was scored as 0 or 1, and adequate core tissue as 2 (Table 1). The tumor area was measured using a digital slide scanner and slide viewer software dedicated to histological assessment (CaseViewer; 3DHISTECH Ltd., Budapest, Hungary). The surface area of adequate core tissue on each scanned slide was digitally marked and calculated using the slide viewer software (Fig. 2). The histologic result of EUS-FNB or surgical resection was the final diagnosis for estimating the diagnostic accuracy.
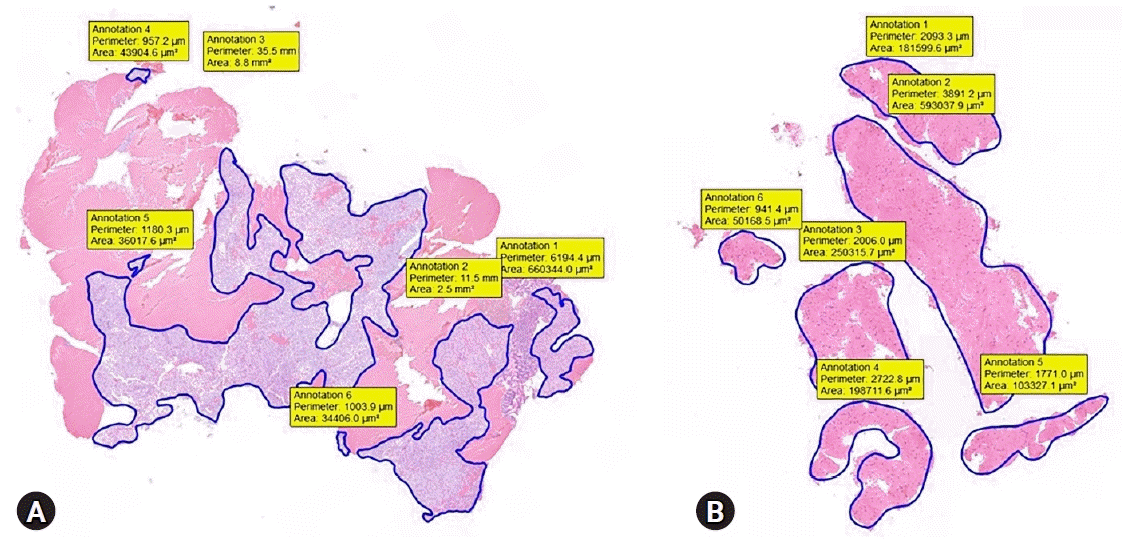
Representative images of the measured tumor area. (A) Gastrointestinal stromal tumor in the gastric corpus. (B) Leiomyoma in the esophagus.
The primary outcome of this study was to compare the adequate tissue acquisition rate and diagnostic accuracy between the SP and SS techniques for EUS-TS in upper GI SETs. The secondary outcome was to determine the factors related to core tissue adequacy using both techniques.
Statistical analysis
Chi-square or Fisher exact tests were performed for categorical variables and were presented as absolute values and percentages. Continuous data were compared using Student t-test or Wilcoxon signed-rank tests and were summarized as mean and standard deviation (SD). A two-sided p-value of <0.05 was considered statistically significant. All analyses were performed using the IBM SPSS ver. 22.0 (IBM Corp., Armonk, NY, USA).
Ethical statements
The study protocol was approved by the Institutional Review Board (IRB) of Kyungpook National University Chilgok Hospital (IRB No: KNUCH 2020-04-044). Informed consent was waived because of retrospective studies.
RESULTS
Baseline characteristics of enrolled patients
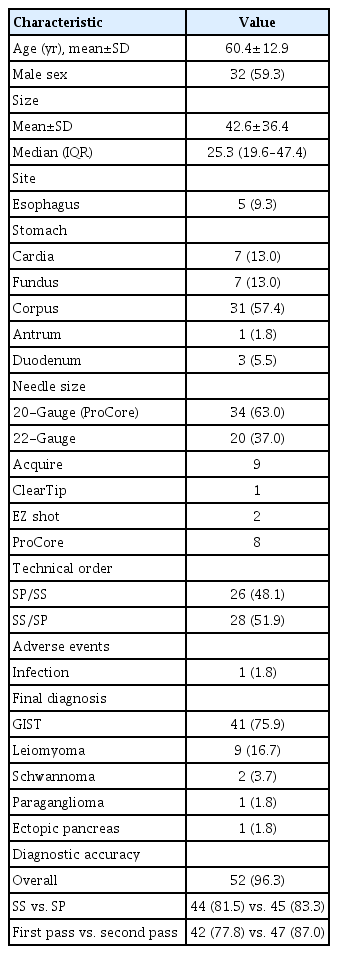
Fifty-four patients were included in this analysis. Most lesions were located at the stomach (85.2%). The mean lesion size was 42.6±36.4 mm. The FNB needles used were ProCore (n=42, 77.8%), Acquire (n=9, 16.7%), EZ shot (n=2, 3.7%), and ClearTip (n=1, 1.8%). In addition, 20- and 22-gauge FNB needles were used in 63% (n=34) and 37% (n=20) of the patients, respectively. The final diagnoses included GIST (n=41, 75.9%), leiomyoma (n=9, 16.7%), schwannoma (n=2, 3.7%), paraganglioma (n=1, 1.8%), and ectopic pancreas (n=1, 1.8%). The proportions of technical orders were 48.1% (SP/SS) and 51.9% (SS/SP). One patient developed an infection related to the procedure and recovered with intravenous antibiotics (Table 2).
Comparison of diagnostic outcomes between slow-pull and standard suction techniques
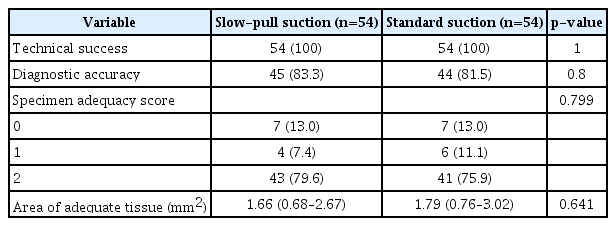
The overall diagnostic accuracies of the SP and SS techniques were 83.3% and 81.5%, respectively (p=0.800). There was no significant difference in the acquisition rates of adequate core tissue between the SP and SS techniques (79.6% and 75.9%, respectively; p=0.799). In addition, there was no significant difference in the median (interquartile range, IQR) tumor area estimated from adequate core tissue between the two techniques (SP, 1.66 [IQR, 0.68–2.67] vs. SS, 1.79 [IQR, 0.76–3.02]; p=0.641) (Table 3).
Subgroup analysis of adequate core tissue rate and tumor area
We performed a subgroup analysis comparing the capability of SP and SS techniques in obtaining an adequate core tissue and the tumor area (mm2) (Figs. 3, 4). We further categorized the descriptors into the location of the lesion (stomach), lesion size (3 cm), FNB needle size, and final diagnosis with each technique. The rate of obtaining adequate core tissue was higher for the SP technique with a 20-gauge FNB needle (SP, 82.4% vs. SS, 67.6%; p=0.161) and for the SS technique with a 22-gauge FNB needle (SP, 75.0% vs. SS, 90.0%; p=0.212); however, there were no significant differences.
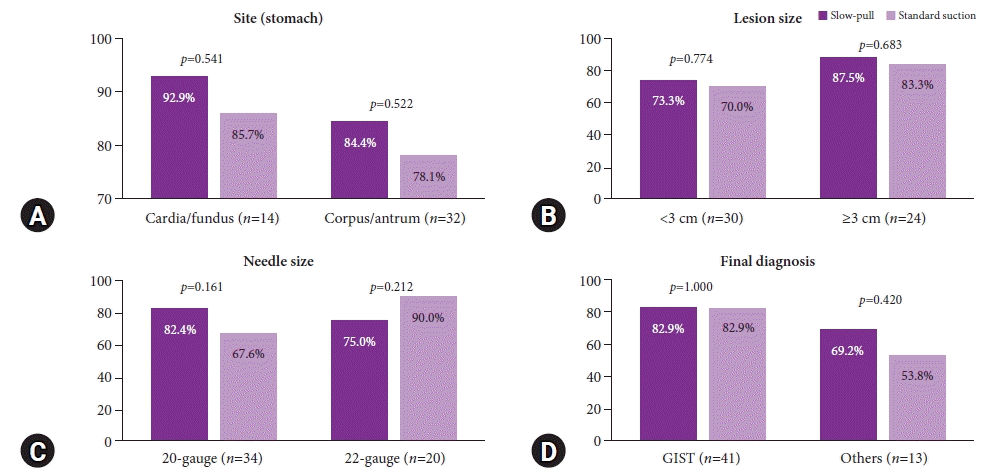
The comparison of adequate core-tissue acquisition rate based on tissue sampling techniques. (A) Lesions located in the stomach. (B) Lesion size. (C) Needle size. (D) Final diagnosis. Others are leiomyoma, schwannoma, paraganglioma, ectopic pancreas. GIST, gastrointestinal stromal tumor.
DISCUSSION
Our study demonstrated that SP and SS have similar diagnostic accuracies (83.3% vs. 81.5%; p=0.800) and comparable efficacies for acquiring adequate core tissue (79.6% vs. 75.9%; p=0.799). In addition, the overall diagnostic accuracy for GI SETs was 96.3%, which showed a higher rate of diagnostic outcomes than a previous meta-analysis that reported 59.9% for EUS-FNB TS in the diagnosis of GI SETs.26 We believe that the difference in diagnostic rates between the previous meta-analysis and our study may be attributed to the presence of inter-study heterogeneity.
EUS-FNB may be a better procedure than EUS-FNA; however, it has suboptimal diagnostic accuracy. This is associated with specimen adequacy and preserved tissue architecture. Most GI SETs have spindle cell features; therefore, IHC staining of the core tissue specimens is necessary. Our general data revealed factors that may improve diagnostic accuracy. In theory, suction may increase the amount of tissue sampled. But the technique increases blood contamination and damages the cell structure. Therefore, the role of suction remains controversial. In fact, most reports have analyzed these factors and their association in pancreatic lesions or other non-GI SET lesions.21,23,27 Consequently, a comparative study between SP and SS for EUS-FNB was required, particularly in GI SETs. Our results suggest that SP and SS have comparable diagnostic outcomes, including rates of obtaining an adequate core tissue and a tumor area.
Several researchers have assessed the diagnostic outcomes using different FNB needle sizes. One retrospective study reported that a definitive diagnosis with full histological assessment, including IHC, is achieved in approximately 88% of cases.28 Additionally, a prospective study assessing the use of a 20-gauge FNB needle (ProCore) showed that the core tissue procurement and diagnosis rates with two needle passes were 94.4% and 88.9%, respectively.29 Similar to a previous study, our findings showed that the 20G ProCore FNB needle had an overall diagnostic accuracy of 91.2% (31/34). However, there was no statistical difference between the SP (79.4%) and SS groups (73.5%; p=0.776). Another retrospective study reported that EUS-FNB using a 22-gauge FNB needle (Acquire) in the diagnosis of pancreatic or GI SETs had a specimen adequacy for ROSE of 96.6% and a diagnostic accuracy of 96.7%.30 One prospective study revealed a 75% yield for diagnosis of GI SETs with the use of a 22-gauge FNB needle (ProCore).31 Our results showed a higher rate for obtaining adequate core tissue with a 20-gauge FNB needle using the SP technique. In contrast, the SS technique showed a higher rate of obtaining adequate core tissue using a 22-gauge FNB needle. However, the differences were not statistically significant.
In contrast, the obtained tumor area showed different results; a larger FNB needle (20-gauge) had a higher capability of acquiring the tumor area with the SS technique. The tumor area was estimated by calculating the sum of the measured adequate core tissues, which might have caused this discrepancy. To the best of our knowledge, no previous reports have compared the SP and SS techniques with different FNB needle sizes. The sampling technique and FNB needle size may have affected the results, since most GISTs have necrotic tissue.32
This study has several strengths. The SP and SS techniques were performed randomly and in an alternative order. This minimized bias from the retrospective design. In addition, we used a scoring system to estimate core tissue adequacy, which could have increased the objectivity of the results. This study had some limitations as well. First, it was a retrospective study. Although the technical order was randomized, the type and size of the FNB needles were not randomized. Second, we performed TS using different FNB needle types and sizes. Third, the bloodiness of EUS-FNB specimens were not compared according to EUS-TS techniques, although continuous negative suction in SS may aspirate more blood than SP. Future studies should estimate the clinical outcomes of TS techniques with regard to different FNB needle sizes and types.
In conclusion, we found comparable diagnostic outcomes of EUS-TS with SP and SS for upper GI SETs in terms of technical success, diagnostic accuracy, and core tissue acquisition rates. These results suggest that both SP and SS are practical options for EUS-guided TS for upper GI SETs.
Notes
Conflicts of Interest
The authors have no potential conflicts of interest.
Funding
None.
Author Contributions
Conceptualization: JSL, CMC, YHK; Data curation: JSL, ANS, MHH; Formal analysis: JSL, CMC; Investigation: JSL, ANS, HIB; Methodology: JSL, CMC, YHK; Project administration: JSL, CMC, YHK; Validation: CMC, YHK; Writing–original draft: JSL; Writing-review & editing: all authors.