A subepithelial lesion in a patient with long-standing ulcerative colitis
Article information
Quiz
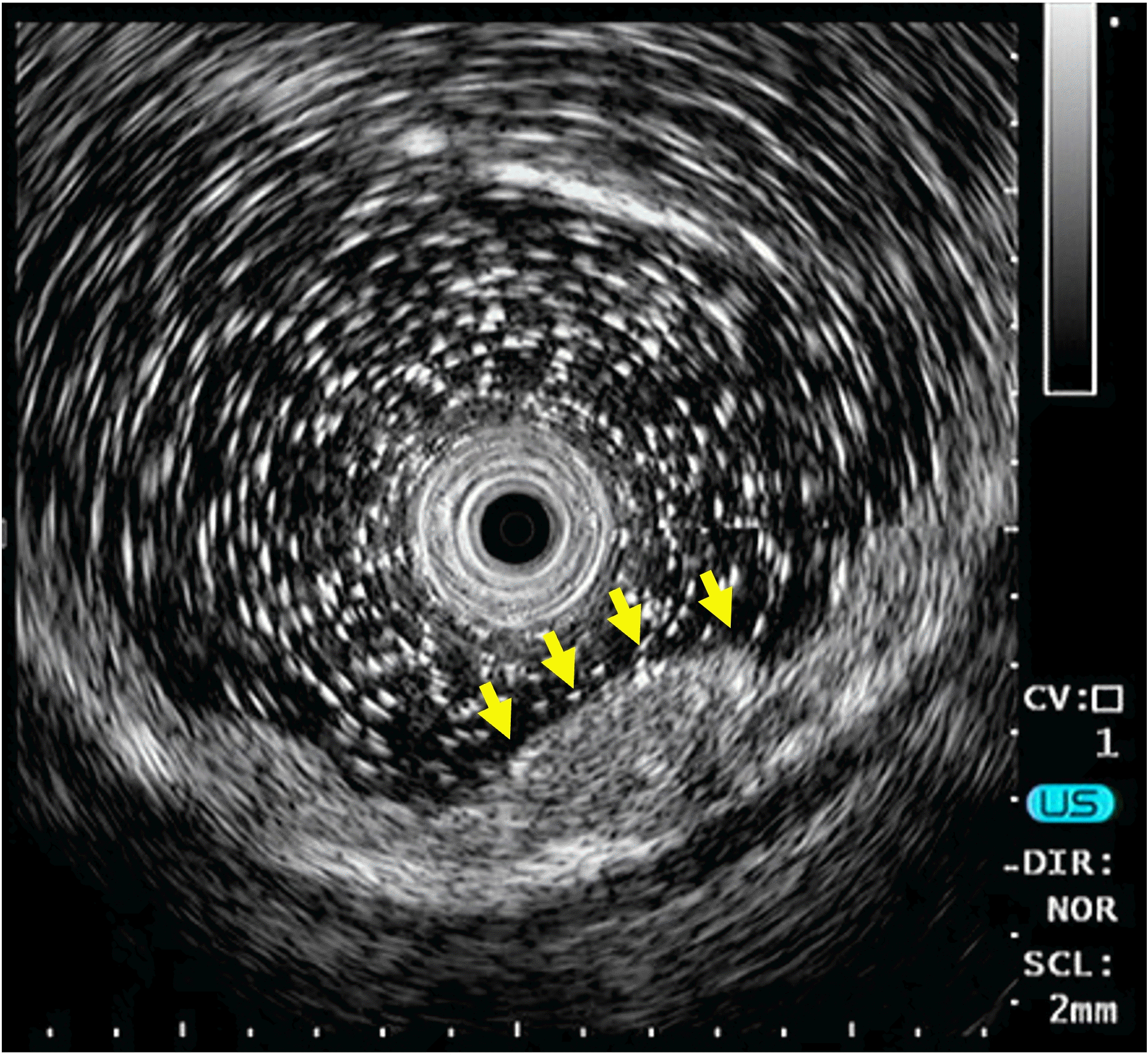
A 59-year-old woman underwent surveillance colonoscopy for extensive ulcerative colitis (UC) diagnosed 20 years ago. She underwent colonoscopies annually for the last 5 years, but colorectal neoplasia was not observed. Colonoscopy revealed a broad spectrum of inflammatory lesions in whole segments, ranging from scar changes to active ulcerations. Additionally, a small subepithelial lesion was detected in the proximal rectum. The surface of this lesion showed mild erythematous changes (Fig. 1). On endoscopic ultrasonography (UM-3R; Olympus Medical, Tokyo, Japan), a 9.5×4.7 mm hypoechoic tumor was detected in the second and third layers of the rectal wall (Fig. 2). What is the most likely diagnosis?
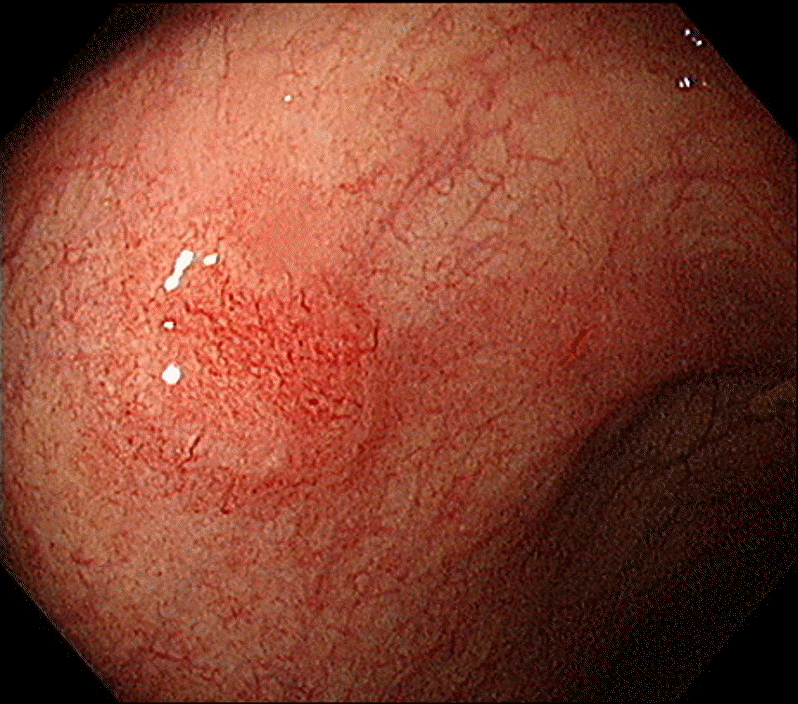
Colonoscopy reveals an erythematous subepithelial lesion at the proximal rectum in the background of diffuse scar change.
Answer
A well-differentiated adenocarcinoma was suspected on the basis of the initial forceps biopsy specimen (Fig. 3A). Because the patient primarily preferred the endoscopic treatment, endoscopic submucosal dissection (ESD) was performed. Histopathological examination of the ESD specimen revealed high-grade dysplasia with clear resection margins (Fig. 3B). However, forceps biopsy specimens obtained from a suspected subepithelial lesion near the post-ESD ulcer were identified as synchronous well-differentiated adenocarcinoma (Fig. 4). Therefore, the patient underwent total proctocolectomy. Pathological examination of the colectomy specimen confirmed the presence of two foci of well-differentiated adenocarcinoma invading the deep submucosal layer (sm3 level) of the rectum without lymph node metastasis (Fig. 5).

Histopathology of the forceps biopsy and endoscopic submucosal dissection specimens. (A) The forceps biopsy specimen shows well-differentiated adenocarcinoma with multifocal irregular glands and desmoplastic stroma (hematoxylin & eosin stain, ×20 objective lens). (B) The endoscopic submucosal dissection specimen shows poorly demarcated dysplasia (upper panel; hematoxylin & eosin staining, ×4 objective lens). A higher magnification of the rectangle clearly shows high-grade dysplasia (lower panel; hematoxylin & eosin staining, ×20 objective lens).

Another synchronous subepithelial lesion was suspected near the endoscopic submucosal dissection site. (A) White light endoscopy image (green arrows). (B) Forceps biopsy specimen shows irregularly shaped glands with marked nuclear pleomorphism that represents well-differentiated adenocarcinoma (hematoxylin & eosin staining, ×20).
Pathologic features of the resected specimen. (A) Two poorly demarcated hyperemic lesions (circles) are noted in the rectum near the distal resection margin. (B) Tumor sections from the upper circle show well-differentiated adenocarcinoma invading the deep submucosa (sm3) with abundant lymphocytic infiltration (hematoxylin & eosin staining, ×10). (C) The second tumor in the lower circle shows more irregular gland shape and thinner neoplastic cells than the first tumor (hematoxylin & eosin staining, ×10). (D) High-grade dysplasia between the two tumors (hematoxylin & eosin staining, ×20).
The risk of colorectal cancer (CRC) is increased in patients with long-standing UC,1 and surveillance colonoscopy for UC is imperative for the timely detection of precancerous dysplasia and CRC at a curable stage. Recent surveillance guidelines recommend targeted biopsy for suspected dysplastic lesions instead of random biopsy in patients with UC.2 Panchromoendoscopy can enhance dysplasia detection in UC compared with white light endoscopy in the standard definition system, and may improve the dysplasia detection rate in the high-definition endoscopy system in patients with UC at a higher risk for CRC.2,3 Moreover, although time-consuming, it can visualize subtle mucosal abnormalities that are not discernible under white light endoscopy. However, in practice, the detection of UC-associated dysplasia or CRC is challenging. Neoplastic pit or narrow band imaging (NBI) patterns help in discriminating dysplasia or cancer in UC; nonetheless, their diagnostic performance seems suboptimal compared with that of pit or NBI patterns for colorectal neoplasia in non-colitic patients.4 Diffuse, chronically inflamed colonic mucosa can confuse endoscopists in discerning the true dysplasia from inflammatory sequelae. Moreover, unlike sporadic colorectal adenoma or cancer, dysplastic changes in colitis-associated lesions occasionally arise from the base of the crypt.5 Consequently, neoplastic pit or NBI patterns may not be obvious on the surface of colitis-associated dysplasia or cancer. Dysplasia and CRC in this case presented as subepithelial lesions on colonoscopy, although the lesions themselves arose from the glandular epithelium. Therefore, during surveillance colonoscopy of patients with UC, targeted biopsies should be performed not only from lesions suspicious of dysplasia or cancer but also from lesions that are inexplicably different from the surrounding mucosa.3
Notes
Conflicts of Interest
Dong-Hoon Yang is currently serving as a section editor of Clinical Endoscopy; however, he had not involved in the peer reviewer selection, evaluation, or decision process of this article. The authors have no potential conflicts of interest.
Funding
None.
Author Contributions
Conceptualization: DHY; Investigations and visualization: SP, JK; Writing–original draft: SP, JK, DHY; Writing–review & editing: SP, JK, DHY.