INTRODUCTION
Obesity is one of the leading causes of morbidity and healthcare-related costs in North America. Among various bariatric surgeries, Roux-en-Y gastric bypass (RYGB) is one of the most commonly performed procedures worldwide.1-4 However, the nature of this surgical procedure, in which the normal sequence of the upper gastrointestinal tract is revised to facilitate weight loss, poses a postoperative challenge for clinicians in choledocholithiasis management, to which this population of patients is particularly susceptible.
Even after percutaneous transhepatic biliary drainage (BD), the current standard of care for choledocholithiasis is endoscopic retrograde cholangiopancreatography (ERCP), in which a duodenoscope is inserted orally and advanced via the stomach into the second part of the duodenum to access the ampulla of Vater for instrumentation of the bile duct. In patients who underwent RYGB, the altered anatomy requires a significant length of the alimentary and biliopancreatic limbs to be traversed before reaching the ampulla; hence, standard ERCP is not possible. Traditionally, this problem has been overcome with other techniques, such as device-assisted (DA) ERCP, including double-balloon or single-balloon enteroscopy and laparoscopy-assisted transgastric (LA) ERCP.
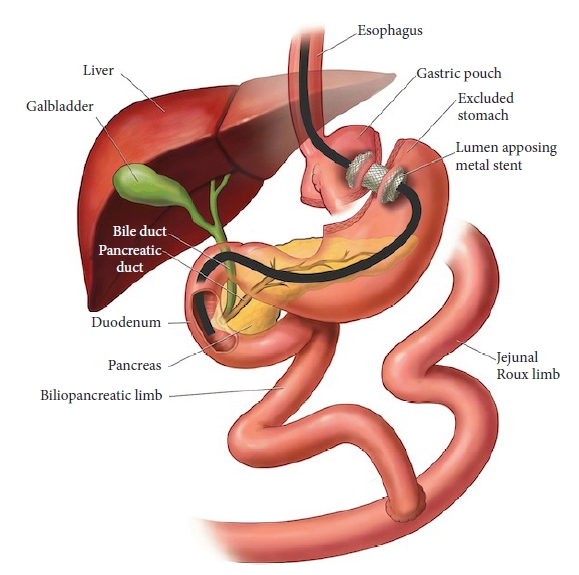
Recently, the increasing application of endoscopic ultrasound (EUS) in therapeutic endoscopy has led to the development of a novel approach, termed endoscopic ultrasound-directed transgastric ERCP (EDGE) (Fig. 1).5 Growing evidence highlights its safety and effectiveness and validates its position as a potential first-line option in these scenarios.6-17
REVIEW OF TECHNIQUE
In this technical review, we describe the EDGE procedure, its indications, technique, outcomes, and complications and compare it to other ERCP methods employed for patients with surgically altered anatomy.
Roux-en-Y gastric bypass anatomy
RYGB surgery for management of morbid obesity was first described in 1994. The surgical steps were as follows: (1) creation of a 75 to 150 cm long bilio-pancreatic limb and a 100 to 150 cm long alimentary or Roux limb (RL); (2) creation of a jejunojejunostomy; (3) creation of a 30-mL gastric pouch from the proximal stomach; and (4) performing a gastrojejunal anastomosis between the end of the RL and the gastric pouch.18,19 Owing to the nature of this operation, approximately 150 to 300 cm of the small bowel lies between the gastric pouch and ampulla, dramatically reducing the chance of successful biliary cannulation even with the use of DA enteroscopes.
Tools and techniques
EDGE is a three-step procedure involving the following processes: (1) EUS-guided placement of a lumen-apposing metal stent (LAMS) for the creation of a gastro-gastrostomy (between the gastric pouch and the remnant stomach) or jejuno-gastrostomy (between the blind limb or RL and the remnant stomach), and (2) using this tunnel, the native stomach is accessed with a duodenoscope and a standard ERCP is performed. To minimize the risk of stent dislodgement, we performed ERCP separately from step 1, allowing the gastro-gastrostomy or jejuno-gastrostomy tract time to mature. (3) Removal of the LAMS and closure of the fistula tract to prevent possible chronic fistula formation and consequent weight gain and/or marginal ulcer formation. No previous studies or reports have recommended the routine use of antibiotics for EDGE. In our center, the administration of antibiotics is determined on the basis of the guidelines or the patientŌĆÖs condition, such as the presence of cholangitis.20,21 Carbon dioxide should be utilized instead of room air in all steps of EDGE.22
1) EUS-guided LAMS placement
Depending on the patientŌĆÖs risk profile, this step can be performed under sedation or general anesthesia. The patient is placed in a left lateral decubitus position. Fluoroscopy is performed in all cases. A therapeutic linear echoendoscope (GF-UCT190; Olympus, Central Valley, PA, USA) is inserted orally and advanced into the gastric pouch or proximal RL to identify the remnant stomach under the sonographic view. On endoscopic ultrasonography, the remnant stomach is often seen as a collapsed cavity with longitudinal folds. Efforts must be made to distinguish it from the neighboring loops of the small intestine, which often have a similar appearance. The tip of the echoendoscope is then manipulated to identify a safe window for the stent insertion using Doppler to avoid damaging any vascular structures in the predicted path of the needle. Ideally, the distance between the two stomach walls should be less than 1 to 2 cm to minimize the risk of separation after needle puncture, which would compromise stent placement. Once an optimal position is confirmed, the remnant stomach is punctured using a 19-gauge fine-needle aspiration (FNA) needle. The stylet is then removed, and the contrast agent is injected under fluoroscopy to confirm that the tip of the needle is within the lumen of the excluded stomach. This is followed by an injection of 100 to 200 mL of sterile water or saline into the excluded stomach to distend it and create a safe target for LAMS placement.
Two types of LAMS are used for gastro-gastrotomy or jejuno-gastrostomy: the non-cautery-tip LAMS or the electrocautery-enhanced LAMS. Based on the previous reports and studies, an electrocautery-enhanced stent (Hot-AXIOS; Boston Scientific, Marlborough, MA, USA) has been shown to have a higher technical success rate with minimal adverse outcomes because of its ability to simplify the steps required for the creation of the fistula tract.23-25 When a non-cautery-tip LAMS is used, a 0.035-inch wire is advanced through the FNA needle and coiled within the remnant stomach. A fistula is then created between the two lumens and dilated with a balloon. The LAMS delivery catheter is advanced over the wire into the remnant stomach and deployed under fluoroscopic and EUS guidance to saddle the two lumens. When an electrocautery-enhanced LAMS is used, the freehand method (without a wire) is feasible for placement. Once the excluded gastric lumen has been adequately expanded, the FNA needle is removed. The LAMS catheter then punctures the excluded stomach, and the LAMS is deployed.
Regardless of the technique used, the diameter of the LAMS should be at least 15 mm to avoid leakage and dislodgement and allow the passage of a duodenoscope for subsequent ERCP. In our center, we typically use a LAMS with a diameter of 15 or 20 mm. Confirmation of the adequate position of the LAMS using contrast-assisted fluoroscopy concludes the first part of EDGE.
2) Standard ERCP
Following the creation of a gastro-gastrostomy or jejuno-gastrostomy, a therapeutic duodenoscope (TJF-Q190V; Olympus) was orally advanced into the gastric pouch, and the proximal opening of the LAMS was visualized. If the lumen of the LAMS has not sufficiently expanded to allow passage of the duodenoscope, the stent lumen is dilated by up to 15 or 20 mm using a balloon dilator (CRE wire-guided balloon; Boston Scientific). The duodenoscope is then advanced through the LAMS, remnant stomach, pylorus, and duodenum to the papilla. Moreover, recent reports described the feasibility of interventional EUS, such as EUS-guided BD and EUS-fine-needle biopsy, and the insertion of duodenal stents through the fistula created by EDGE.16 Once the procedure is complete, preemptive placement of a guidewire into the duodenum is recommended to reduce the risk of LAMS dislodgement during scope withdrawal.11 This very simple but useful technique provides a safety net for the endoscopist to re-access the excluded stomach over the guidewire in case of dislodgement of the LAMS and separation of the two lumens.
3) Fistula closure
The LAMS should be removed four weeks after its placement to allow for adequate maturation of the gastro-gastrostomy or jejuno-gastrostomy tract.26 Grasping forceps or a snare can be used for removal of the LAMS, and closure of the fistula is managed at the discretion of the endoscopist using standard clips, over-the-scope clips, or endoscopic sutures. Argon plasma coagulation can be applied to the fistula tract prior to the closure to denude the mucosa of the tract and enhance tissue apposition of the fistula.27 In the cases where it is deemed advantageous to maintain the fistula tract for future use, exchanging the LAMS for plastic double pigtail stents have been recently reported as an option.28,29 Once no longer required, the fistula can be closed using the same techniques described above after the pigtail stents have been removed.
Recent studies have reported the use of EDGE for permanent reversal of RYGB in patients with pancreaticobiliary malignancy to facilitate endoscopic interventions and to combat the rapid weight loss experienced by these patients during chemotherapy or palliative care.11
Technical tips
1) Access point
A gastrostomy tract (between the gastric pouch and remnant stomach body) is the preferred access point for EDGE owing to its lower risk of dislodgement and faster maturation than a jejuno-gastrostomy tract.29 Occasionally, detecting and/or accessing the remnant stomach from the gastric pouch is difficult. In this situation, expansion of the remnant stomach after injecting 100-200 ml of sterile water or saline into the jejunum may be more appropriate.
2) Procedure intervals
The optimal interval between EUS-guided LAMS placement and ERCP is determined by the position of the LAMS and the urgency of the indication. In the setting of urgent indications, ERCP can be performed immediately after LAMS placement in the same session. For less urgent indications, at least 5 to 7 days interval is preferable to allow for tract maturation and reduce the risk of LAMS dislodgement.8 For the patients with gastro-gastrostomy tract, recent studies reported that a single session procedure is safe if the LAMS flanges are fixed well against the gastric wall.10,12,29 For non-urgent patients with a jejuno-gastrostomy tract, the timing of the ERCP session should be 2 weeks following LAMS placement because the thinner and more pliable jejunal wall takes longer to mature.29
Complete maturation of the tract requires approximately 4 weeks.26 Therefore, LAMS removal and fistula closure should be performed 4 weeks after the initial LAMS placement, as earlier attempts may result in free perforation of the remnant stomach, which may require urgent surgical closure.
OUTCOMES
1) Success rate
A recent meta-analysis compared different ERCP modalities in patients undergoing RYGB surgery. EDGE and LA-ERCP have higher technical and clinical success rates than DA-ERCP. Technical success rates were 95.5%, 95.3%, and 71.4%, respectively, while clinical success rates were 95.9%, 92.9%, and 58.7%, respectively.9 If ERCP fails, repeat ERCP or EUS-guided BD should be considered because of the presence of an access route to the remnant stomach.
2) Dislodgement
Dislodgement of the LAMS is one of the major complications of EDGE, reported having an incidence of 13.3% in a recent meta-analysis.9 The main cause of dislodgement is the immaturity of the fistula tract. Most dislodgements are induced by scope movements during the procedure, specifically, scope withdrawal. A jejuno-gastrostomy tract is another well-described risk factor for dislodgement due to the thinner jejunal wall preventing a stable fixation of the LAMS.29,30
The most important measure is maintaining access to the remnant stomach. Recent case reports have described the importance of placing a wire in the duodenum before withdrawal and scope exchange.11,16 This simple step enables the endoscopist to re-access the remnant stomach and reposition the LAMS or re-deploy another LAMS. The strategies to avoid dislodgement of the LAMS are: (1) use of a thinner duodenoscope, (2) creation of a gastro-gastrostomy tract whenever possible, (3) performing the second step of the EDGE 2 to 3 weeks after LAMS placement, (4) using a larger diameter LAMS (20 mm if possible), and (5) use of generous lubrication.29,31,32
3) Persistent fistula
Runge et al.28 reported that 10% of patients who underwent esophagogastroduodenoscopy after LAMS removal had a persistent fistula for at least 8 weeks. In this study, no statistically significant difference was observed between the rates of persistent fistula following gastro-gastrostomy and jejuno-gastrostomy LAMS placement. James and Baron7 reported a chronic fistula six months after LAMS removal. However, such fistulas often close spontaneously with expectant management or can be readily closed using additional endoscopic fistula closure methods.
4) Other complications
Other complications of EDGE reported include bleeding 6.6% (95% confidence interval [CI] 3.3%ŌĆō13%) and perforation 2.2% (95% CI 0.6%ŌĆō7.4%). Post-ERCP pancreatitis rates corresponded to rates described in the literature for standard ERCP at 2.2% (95% CI, 0.6%ŌĆō7.4%).9
DISCUSSION
Pancreaticobiliary access in patients who have undergone RYGB is challenging because of surgically altered anatomy. Currently, there are three major methods for overcoming these challenges: DA-ERCP, LA-ERCP, and EDGE.
Although DA-ERCP is recognized as a safe and non-invasive procedure, its disadvantages include a lower technical success rate, longer procedure time, and the requirement for special, long accessories to perform the ERCP.9,33 Schreiner et al.33 reported that in patients with RYGB anatomy where there is less than 150 cm of the small bowel to traverse (RL and biliopancreatic limb), a DA-ERCP might be offered because this was associated with a higher technical success rate.
A recent meta-analysis illustrated the high technical success rate of LA-ERCP.9 In addition, LA-ERCP allowed for cholecystectomy during the same session if clinically indicated. The technical success rate and adverse event profile of EDGE appear to be equivalent to LA-ERCP (technical success rate, 95.5% vs. 95.3%; adverse events, 21.9% vs. 17.4%).9 There is growing evidence that EDGE may be superior to LA-ERCP based on its cost-effectiveness, procedure time, and length of hospital stay.32 Based on our experience and review of the available literature, we propose the following algorithm to determine the optimal management for these patients (Fig. 2).
CONCLUSIONS
Although EDGE is a technically challenging procedure with a risk of complications, such as dislodgement, recent studies have shown extremely good technical and clinical success rates. Therefore, the EDGE procedure is well-positioned as a potential first-line therapy for managing pancreatobiliary disease in patients with altered gastrointestinal anatomy after RYGB.