AbstractSubmucosal endoscopy or third-space endoscopy utilizes the potential space between the mucosal and muscularis layers of the gastrointestinal tract to execute therapeutic interventions for various diseases. Over the last decade, endoscopic access to the submucosal space has revolutionized the field of therapeutic endoscopy. Submucosal endoscopy was originally used to perform endoscopic myotomy in patients with achalasia cardia, and its use has grown exponentially since. Currently, submucosal endoscopy is widely used to resect subepithelial tumors and to manage refractory gastroparesis and Zenkerās diverticulum. While the utility of submucosal endoscopy has stood the test of time in esophageal motility disorders and subepithelial tumors, its durability remains to be established in conditions such as Zenkerās diverticulum and refractory gastroparesis. Other emerging indications for submucosal endoscopy include esophageal epiphrenic diverticulum, Hirschsprungās disease, and esophageal strictures not amenable to conventional endoscopic treatment. The potential of submucosal endoscopy to provide easy and safe access to the mediastinum and peritoneal spaces may open doors to novel indications and rejuvenate the interest of endoscopists in natural orifice transluminal endoscopic surgery in the future. This review focuses on the current spectrum, recent updates, and future direction of submucosal endoscopy in the gastrointestinal tract.
INTRODUCTIONThe submucosal or third space is a virtual space between the mucosa and muscularis layer that can be expanded using various injectable solutions and accessed endoscopically. The submucosal space provides the opportunity to manage various pathological conditions involving the submucosal and muscular layers and beyond. The concept of submucosal endoscopy originated approximately one and a half decades ago when Sumiyama et al.,1,2 in their seminal work on porcine models, showed that the peritoneal cavity and mediastinum could be successfully accessed via submucosal endoscopy, with the defect being completely sealed using the mucosal flap. The authors coined the term āsubmucosal endoscopy with mucosal flap safety valve (SEMF)ā . Subsequently, Pasricha and colleagues suggested that the submucosal space may be exploited to perform endoscopic esophageal myotomy as a potential treatment in cases of achalasia.3 Inoue and colleagues are credited with performing the first human submucosal endoscopic myotomy in cases of achalasia cardia and coining the term per-oral endoscopic myotomy (POEM).4 Over the last decade, the use of submucosal endoscopy has witnessed exponential growth, and it is now being utilized for a number of gastrointestinal (GI) diseases such as esophageal motility disorders, subepithelial tumors (SETs), Zenkerās diverticulum, and refractory gastroparesis (Fig. 1).5
This review focuses on the current spectrum, recent updates, and future directions of submucosal endoscopy in the GI tract.
CURRENT SPECTRUM OF SUBMUCOSAL ENDOSCOPYEsophagusIn the GI tract, submucosal endoscopy is most widely used in the esophagus. The major indications of submucosal endoscopy in the esophagus include POEM for esophageal motility disorders, submucosal tunneling endoscopic resection (STER) for SETs, submucosal tunneling with endoscopic division of septum in cases of Zenkerās diverticulum (Z-POEM) and epiphrenic diverticulum (diverticular [D]-POEM), and tunneling technique for restoration of the esophagus.
1) Esophageal motility disorders (POEM)POEM has emerged as a safe and effective modality for the palliation of symptoms in cases of achalasia and other non-achalasia esophageal motility disorders such as diffuse esophageal spasm, jackhammer esophagus, and esophagogastric junction outflow obstruction. The POEM technique is based on the principles of SEMF and involves submucosal lifting, mucosal incision, submucosal tunneling, myotomy, and closure of the mucosal incision (Fig. 2). Multiple studies with short-term follow-ups have confirmed the safety and efficacy of POEM in esophageal motility disorders. Major adverse events (AEs) are rare with POEM and range from 0.5% to 3% in large studies using a standardized definition for defining AEs.6-8 The safety and efficacy of POEM has also been established in the pediatric age group,9 elderly population,10 spastic esophageal motility disorders,11 patients with prior treatment failure,12 and those with sigmoid achalasia.13
Emerging data indicate that the response to POEM is durable at long-term follow-ups. The long-term clinical success of POEM in recent studies has ranged from 72% to 96% at follow-up durations of 36 to 120 months (Table 1).13-29 POEM has also been compared to pneumatic dilatation (PD) and Hellerās myotomy (HM) in several retrospective cohort studies, suggesting a similar or even superior efficacy of POEM.30 More recently, two landmark randomized trials compared POEM to PD and HM.31,32 In the randomized trial comparing POEM and PD, POEM was more effective than PD at the two-year follow-up (92% vs. 54%).31 POEM and HM were equally effective after two years in another randomized trial comparing these modalities (83% vs. 82%).32 Of note, the incidence of gastroesophageal reflux disease (GERD) was higher after POEM in both studies (POEM, 41% vs. PD, 7% and POEM, 44% vs. HM, 29%). There are ample data to support the safety and efficacy of POEM in achalasia, and current international guidelines acknowledge the role of POEM as a frontline treatment modality along with PD and HM.33-37
2) Zenkerās diverticulum (Z-POEM)The management options for Zenkerās diverticulum include surgery, rigid endoscopic diverticulotomy, and flexible endoscopic septotomy (FES). Among these, FES has gained widespread acceptance owing to its excellent safety and efficacy. However, symptoms recur in up to a third of patients after FES and are mainly attributed to incomplete division of the cricopharyngeal septum.38-40 More recently, submucosal endoscopy has been described in cases with Zenkerās diverticulum (Z-POEM) with encouraging results. The Z-POEM technique is similar to esophageal POEM for achalasia cardia. Briefly, the steps of Z-POEM include submucosal injection (saline with indigo carmine) 1 to 2 cm proximal to the septum, mucosal incision, submucosal tunneling along the Zenkerās pouch as well as along the esophageal side, division of the septum, and closure of the incision with endoclips (Fig. 3). A crucial modification in the Z-POEM technique (over the septum technique) involves submucosal elevation and mucosal incision on the top of the cricopharyngeal septum. This approach may be technically easier because of space restrictions, which impede the creation of a proximal entry in the conventional approach. Other modifications include hybrid Z-POEM in cases with submucosal fibrosis due to previous treatment and incision of the mucosa after POEM to reduce recurrences arising as a result of a remnant mucosal pouch.41
The proposed advantage of Z-POEM is its ability to completely divide the cricopharyngeal septum, which may reduce recurrence in the future. Moreover, preservation of the mucosa reduces the risk of intraprocedural perforations. Several studies have evaluated the safety and efficacy of Z-POEM in patients with Zenkerās diverticulum.42-49 In these studies, the clinical success rate was 91% to 100% at a mean follow-up of 3 to 10 months (Table 2).42-49 Meanwhile, AEs related to Z-POEM have been reported in 0% to 13.6% of cases. Although there are no randomized trials comparing Z-POEM and FES, a few retrospective comparative studies have suggested that clinical success is comparable between the two techniques.47-49
3) Esophageal epiphrenic diverticulum (D-POEM)Esophageal epiphrenic diverticulum (EED) is a type of diverticulum that develops in the distal part of the esophagus and is typically associated with motility disorders. Surgery is the mainstay of EED management. While surgical options (thoracotomy or laparoscopy) provide symptom relief in most cases, high morbidity, especially leaks, remains an important concern. Submucosal endoscopy has emerged as a minimally invasive treatment option for patients. Broadly speaking, the technique of D-POEM is similar to that described for Zenkerās diverticulum. A key difference is that myotomy of the lower esophageal sphincter is also performed in addition to dividing the septum in selected cases with evidence of high integrated relaxation pressure. Several studies have described the results of D-POEM for EED.50-52 Nabi et al.52 described the outcomes of D-POEM in thirteen cases with EED. A co-existing esophageal motility disorder was evident in three-fourths of cases. At a median follow-up of 25 months, clinical success was achieved in 84.6% of cases. While septotomy appears to be an integral step of D-POEM, several recent studies suggest that myotomy of the lower esophageal sphincter without septotomy may provide adequate symptom relief.53,54 There are no data comparing the two approaches; therefore, it may be prudent to individualize the management approach in these cases. In a recent review, Samanta et al.55 proposed a tailored approach to EED based on the size of the diverticulum and the presence or absence of esophageal motility disorder with a non-relaxing lower esophageal sphincter. The authors suggested that septotomy may be avoided in cases with small diverticula and non-relaxing lower esophageal sphincter. Septotomy should be considered in cases with a large EED without evidence of motility disorder.
4) Refractory esophageal strictures (per-oral endoscopic tunneling for restoration of the esophagus)Complete esophageal obstructions involving a long segment of the esophagus (>3 cm) are difficult to manage using currently available endoscopic techniques. In such cases, Wagh and colleagues reported the utility of submucosal endoscopy to restore the esophagus i.e. per-oral endoscopic tunneling for restoration of the esophagus (POETRE).56,57 In a small series including four cases with complete and long (>3 cm) segment esophageal obstruction, significant improvement in dysphagia was noted in all patients who underwent POETRE.57 Subsequently, other authors have reported the technical feasibility and efficacy of POETRE.58 Briefly, the technique of POETRE is as follows. Initially, the length of the obstructed segment is estimated by calculating the distance between the tips of the endoscopes inserted per-orally and via the gastrostomy site under fluoroscopic guidance. Depending on the location of the stricture, antegrade or retrograde (via the gastrostomy site) tunneling is performed starting a few centimeters proximal to the stricture site. Once the stenotic area is reached, careful dissection of the fibrotic scar tissue is performed until the other end of the stricture is reached. Fluoroscopic visualization of the endoscope at the other end of the stricture provides guidance during the dissection of fibrotic tissue. The aim of submucosal endoscopy in these cases is to traverse the strictured segment, after which a fully covered self-expandable metal stent is placed across the stricture. This restores the patency of the esophagus and allows the initiation of oral feeding. The metal stent is removed after 3 to 4 weeks. Subsequently, esophageal dilatations are performed at regular intervals to maintain the patency of the esophagus.
StomachThe major indications for submucosal endoscopy in the stomach include resection of SETs and management of refractory gastroparesis.
1) Refractory gastroparesis (gastric POEM or per-oral endoscopic pyloromyotomy)A sizable proportion of patients with gastroparesis do not respond to conservative treatment, such as optimization of glycemic control, dietary modifications, and prokinetics, and are classified as having refractory gastroparesis. The management of refractory gastroparesis is challenging and often unsatisfactory. The frontrunners in the management of refractory gastroparesis include gastric electrical stimulation (GES) and laparoscopic pyloroplasty, which has been shown to be a safe and effective treatment options in recent studies.59,60 Encouraging results with surgical pyloroplasty propelled the evaluation of per-oral endoscopic pyloromyotomy (POP) or gastric POEM (G-POEM) in the management of refractory gastroparesis.
The technique of G-POEM is essentially similar to that of esophageal POEM and involves mucosal incision, tunneling, and myotomy of the pyloric sphincter (Fig. 4). The conventional G-POEM technique involves a greater curvature approach. Modifications in technique include a lesser curvature approach to pyloromyotomy and performing double pyloromyotomy instead of single pyloromyotomy.61,62 The advantages of modified techniques over and above the conventional technique remain to be seen in controlled trials. Overall, G-POEM is a safe procedure, and most AEs are mild (abdominal pain, mucosotomy, and capnoperitoneum).63
Multiple studies with short-term follow-up suggest the safety and efficacy of G-POEM (Table 3).64-69 In a systematic review including ten studies (292 patients), symptomatic improvement was achieved in 83.9% of cases at a pooled mean follow-up duration of 7.8Ā±5.5 months.70 However, there are limited data on the long-term outcomes of G-POEM. Some of the recent studies have indicated that the efficacy of G-POEM may decline at a longer follow-up duration i.e., >1 year. In these studies, the clinical efficacy of G-POEM ranged from 48% to 69% at the one-year follow-up.64-69 The modest efficacy of G-POEM suggests the urgent need to determine the predictors of response after G-POEM to optimize its use in clinical practice. In a well-conducted, prospective, multicenter study, a baseline gastric cardinal symptom index score >2.6, gastric retention >20% at 4 hours, and early response to G-POEM at 1 month after therapy were independent predictors of clinical success at 12 months.68 In another prospective multicenter study, a predictive score was devised using several factors, including nausea, early satiety, bloating, and gastric retention at 4 hours on scintigraphy.69 Patients with scores ā„2 were significantly more likely to be responders at 3 years than patients with scores <2 (80% and 18%, respectively; p=0.001). The predictors of clinical success or failure identified in these studies need to be validated in future studies.
2) Subepithelial tumors (STER)Endoscopic resection techniques for SETs in the upper GI tract include endoscopic submucosal dissection, endoscopic submucosal excavation (ESE), endoscopic full-thickness resection, and STER. Of these, STER utilizes the principles of SEMF for resection of upper GI SETs (Fig. 5). The safety and efficacy of STER for SETs were confirmed in a recent systematic review and meta-analysis wherein the complete resection and en-bloc resection rates were 97.5% and 94.6%, respectively.74 Major AEs were uncommon, and minor AEs included insufflation-related AEs (14.8%) and perforation (5.6%).
Several studies have compared STER with other treatment modalities, such as ESE and video-assisted thoracoscopic surgery (VATS). In retrospective comparison studies, STER has been equally effective to ESE and VATS.75 The advantages of STER over ESE include preservation of mucosa, reduced rate of perforation, and easier closure of the mucosal defect. However, STER may be associated with a longer operating time and may be unsuitable for tumors located in the distal fundus or lesser curvature (Fig. 6).76,77 In studies comparing STER to VATS for esophageal SETs, STER has been associated with a shorter procedure duration, lower cost, and shorter hospital stay.78,79
In conclusion, an individualized approach is warranted for esophagogastric SETs. ESE may be preferred to STER in tumors located in the distal fundus or lesser curvature, and VATS may be a better option for esophageal SETs with a minor axis diameter >30 mm or tumor mass index (major axisĆminor axis) >1,000.79,80
Rectum1) Hirschsprungās disease (per-rectal endoscopic myotomy)Hirschsprungās disease (HD) or congenital megacolon results from the failed migration of colonic ganglion cells, resulting in the inability of a colonic segment of varying length to relax, leading to functional colonic obstruction. The aganglionic segment is usually localized in the rectosigmoid region; therefore, an endoscopic approach appears intuitive in HD. Bapaye et al.81 reported the use of submucosal endoscopy in nine cases with HD and coined per-rectal endoscopic myotomy (PREM).81 In their study, the mean length of the aganglionic segment was 6.3 cm, and all patients successfully underwent PREM with a mean procedure duration of 96 minutes and no major intraoperative AEs. At a median follow-up of 17 months (range, 9ā58 months), the stool frequency improved and the requirement for laxatives was reduced in all cases.
The PREM technique is illustrated in Figure 7. First, the length of the aganglionic segment is estimated using serial biopsies at 2ā3-cm intervals in the rectum. The technique of endoscopic biopsy has previously been described by the authors of this review.82,83 The subsequent steps are similar to esophageal POEM and include mucosal incision and submucosal tunneling until the predetermined extension of the aganglionic segment followed by myotomy and closure of the mucosal incision with endoclips (Fig. 7). Caution is advised while performing myotomy close to the anal verge to avoid inadvertent damage to the external anal sphincter. Barring this study, the data are limited regarding the utility of PREM in HD. Therefore, large-scale studies with long-term follow-ups are required before PREM can be advocated in routine clinical practice.
SUBMUCOSAL ENDOSCOPY: RECENT ADVANCESThe field of submucosal endoscopy is over a decade old. However, new information and advancements are constantly enriching the wisdom of third-space endoscopists. In this section, we discuss the recent progress in submucosal endoscopy (Table 4).
While the safety and efficacy of POEM have been established in studies published over the last decade, more recent studies have focused on the efficacy of short esophageal myotomy,84-87 prevention of postoperative GERD, and the utility of the endoluminal functional lumen imaging probe (EndoFLIP) in predicting the outcomes of POEM.88-91
The length of esophageal myotomy during POEM is usually 6 to 8 cm based on the initial description of the procedure by experts. However, the same is not evidence-based, and recent studies have questioned the utility of long esophageal myotomies in achalasia. Several randomized trials and systematic reviews have concluded that short esophageal myotomy (3ā5 cm) is equally effective, with the advantage of reduced procedure duration and possibly less esophageal acid exposure as compared to those associated with standard esophageal myotomy.92 In a recent study, the distensibility index (DI) of the lower esophageal sphincter fell within the target range for most patients following a 2ā4-cm esophageal myotomy, further supporting the efficacy of short esophageal myotomy in patients with achalasia.93
GERD is the most common long-term AE of POEM. Emerging data suggest that although the incidence of post-POEM GERD is high, most patients respond to anti-secretory medications.91 In addition, the incidence of GERD may decrease with time due to remodeling of the gastroesophageal junction.26 Several new approaches have been suggested to address the issue of GERD after POEM. These include modification of POEM techniques (preservation of sling fibers), natural orifice transluminal endoscopic surgery (NOTES) fundoplication, and trans-oral incisionless fundoplication after POEM.94-98 Quality trials are required before confirming the efficacy of these methods in preventing or treating post-POEM GERD.
The EndoFLIP system has recently been introduced in clinical practice to predict the clinical outcomes of POEM in achalasia and refractory gastroparesis. It utilizes impedance planimetry to determine the DI and cross-sectional area of the gastroesophageal junction or pyloric sphincter. The current literature is divergent with regard to the utility of DI and cross-sectional area in predicting the outcomes of POEM and G-POEM in esophageal motility disorders and refractory gastroparesis, respectively.99,100 Therefore, future studies are required to determine the real-world utility of EndoFLIP in GI motility disorders.
SUBMUCOSAL ENDOSCOPY: FUTURE PERSPECTIVESOver the last decade, submucosal endoscopy has proven useful for a broad spectrum of GI diseases. Of note, submucosal endoscopy is an evolving field, and the certainty of evidence and efficacy varies across different indications in the GI tract. While the safety and efficacy of submucosal endoscopy have been consistent across the indications, the durability of response needs to be established for some indications, such as refractory gastroparesis and Zenkerās diverticulum. Additionally, evidence of its efficacy is limited in HD and esophageal strictures. With continued advancements in the field of submucosal endoscopy, there may be renewed interest in NOTES in the future. This is exemplified by recent reports of endoscopic transcolonic appendicectomy and transgastric cholecystectomy.101,102 In addition, endoscopists are likely to exploit the easy access to the third space or submucosal space when performing muscle biopsies to gain a better understanding of the pathophysiology of esophageal and gastric motility disorders.103-106 Similarly, safe access to the mediastinal and peritoneal spaces may potentially expand the indications of the ever-growing field of submucosal endoscopy.107,108
CONCLUSIONSSubmucosal endoscopy has been one of the most rewarding innovations in the field of therapeutic endoscopy in the recent era. Beginning with achalasia, the submucosal space is now being utilized to manage several GI diseases. While the utility of submucosal endoscopy has stood the test of time in esophageal motility disorders and SETs, its durability remains to be established in other conditions such as Zenkerās diverticulum and refractory gastroparesis. Additionally, novel techniques are required to reduce the incidence of GERD after POEM. Further studies are required to identify the predictors of response to G-POEM in patients with refractory gastroparesis. The potential of submucosal endoscopy to provide easy and safe access to the mediastinal and peritoneal spaces may open doors to novel indications and rejuvenate the interest of endoscopists in NOTES in the future.
Fig.Ā 2.Technique of per-oral endoscopic myotomy in esophageal motility disorders. (A) Submucosal lifting injection using an injection needle. (B) Mucosal incision using a triangular knife. (C) Submucosal tunneling using a triangular knife. (D) Control of intraprocedural bleeding using coagulation forceps. (E) Selective circular (upper part) and full-thickness myotomy. (F) Closure of the mucosal incision with endoclips. 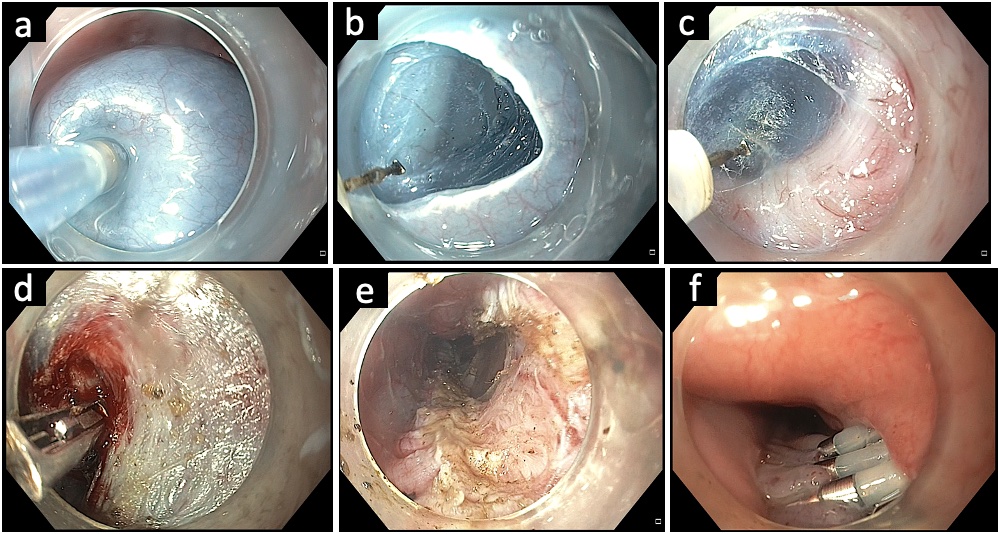
Fig.Ā 3.Submucosal tunneling with division of cricopharyngeal septum in a case of Zenkerās diverticulum. (A) Endoscopic image revealing the cricopharyngeal septum. (B) Submucosal lifting injection proximal to the septum. (C) Mucosal incision using an electrosurgical knife. (D) Submucosal tunneling along the diverticulum pouch. (E) Submucosal tunneling along the esophageal side and complete exposure of the septum. (F) Division of the cricopharyngeal septum using an electrosurgical knife. (G) Completion of cricopharyngeal myotomy. (H) Closure of mucosal incision with multiple endoclips. 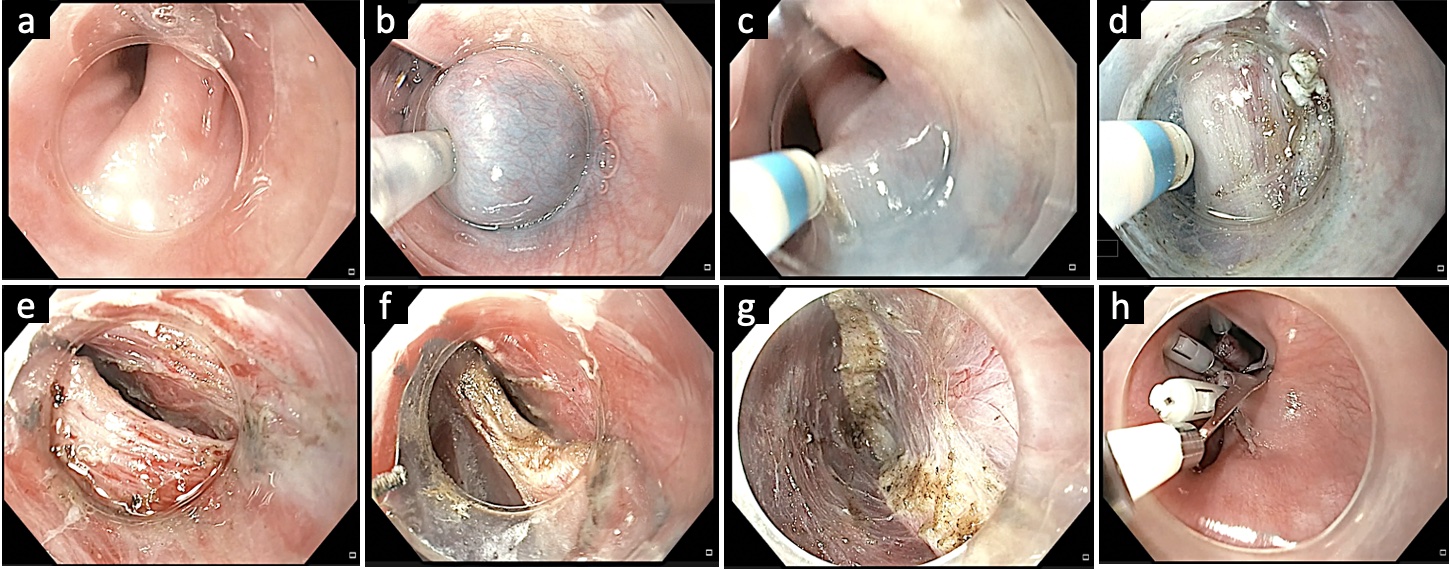
Fig.Ā 4.Gastric per-oral endoscopic myotomy in a case of refractory gastroparesis. (A) Submucosal lifting injection 3 to 4 cm proximal to the pylorus. (B) Submucosal tunneling towards the pylorus. (C) Visualization of the pyloric sphincter. (D) Confirmation of extension of submucosal tunnel beyond the pyloric sphincter. (E) Execution of endoscopic pyloromyotomy. (F) Endoscopic appearance of the pyloric sphincter after pyloromyotomy. Courtesy by Harshal Mandavdhare (Associate Professor, Post Graduate Institute of Medical Education and Research, Chandigarh, India). 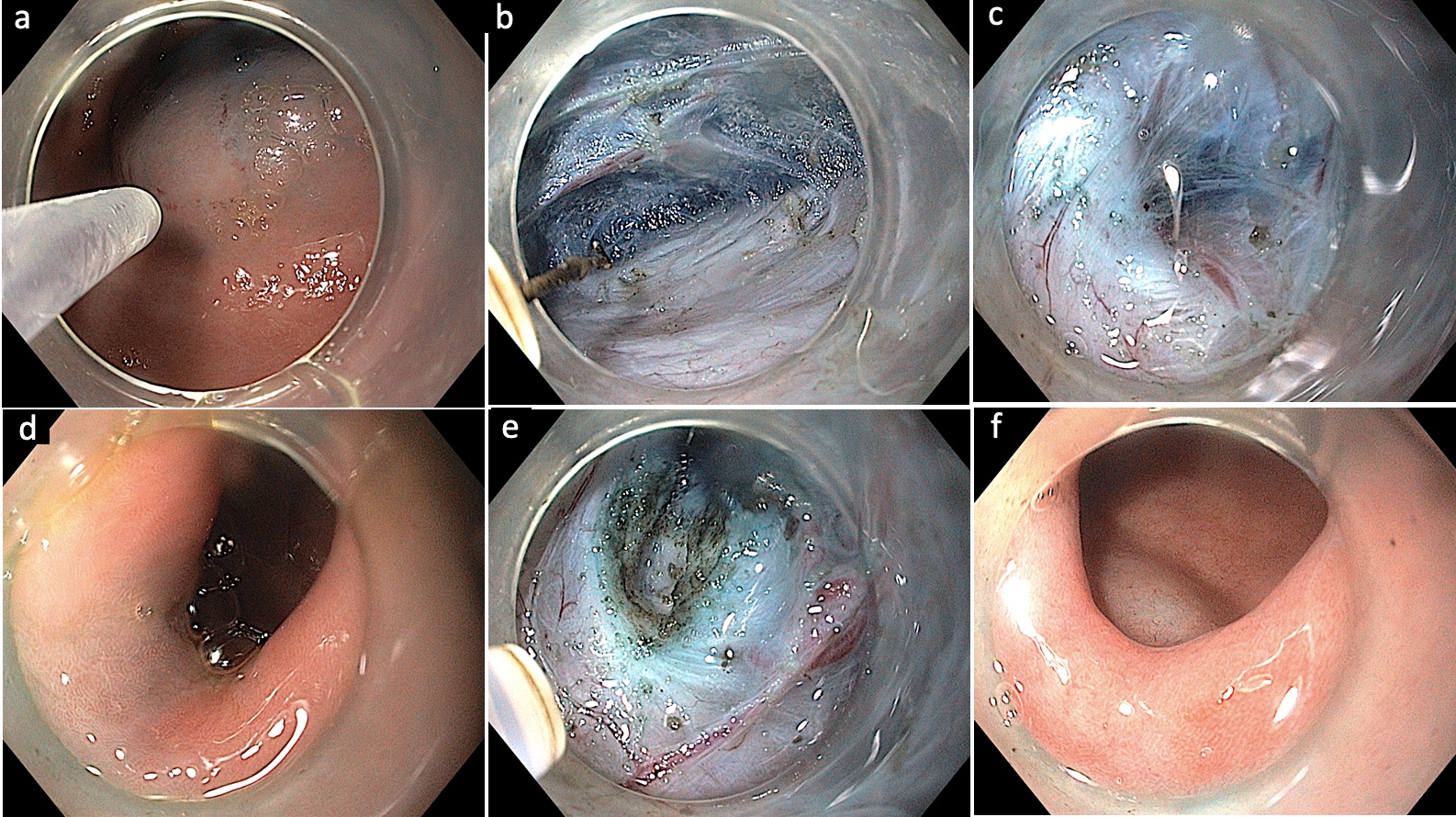
Fig.Ā 5.Submucosal tunneling endoscopic resection in a case of esophageal subepithelial tumor. (A) Endoscopic appearance of the mid-esophageal subepithelial lesion. (B) Submucosal lifting injection 2 to 3 cm above the subepithelial lesion. (C) Vertical mucosal incision using a triangular knife. (D) Submucosal tunneling along the presumed orientation of the subepithelial lesion. (E) Exposure of the subepithelial tumor. (F) Dissection of the tumor from surrounding attachments. (G) Retrieval of the tumor using a polypectomy snare. (H) Closure of the mucosal incision using endoclips. 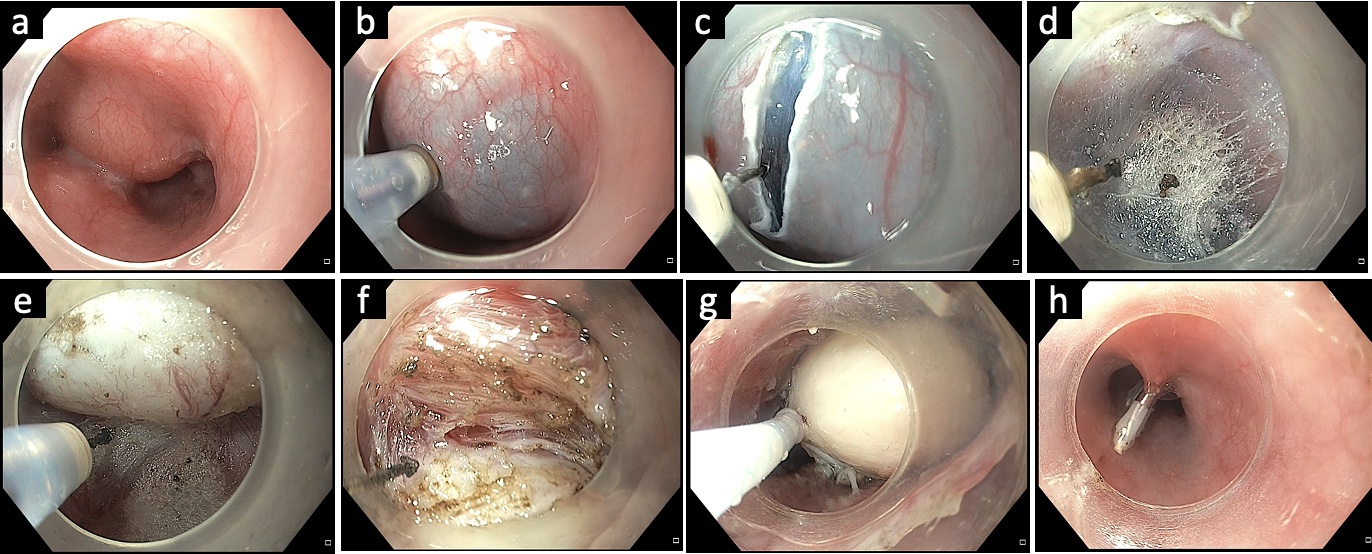
Fig.Ā 6.Individualized approach to gastric subepithelial. Note the preferred location of gastric submucosal lesions for ESE and STER (from Lu et al. PLoS One 2015;10:e011987077). ESE, endoscopic submucosal excavation; STER, submucosal tunneling endoscopic resection. 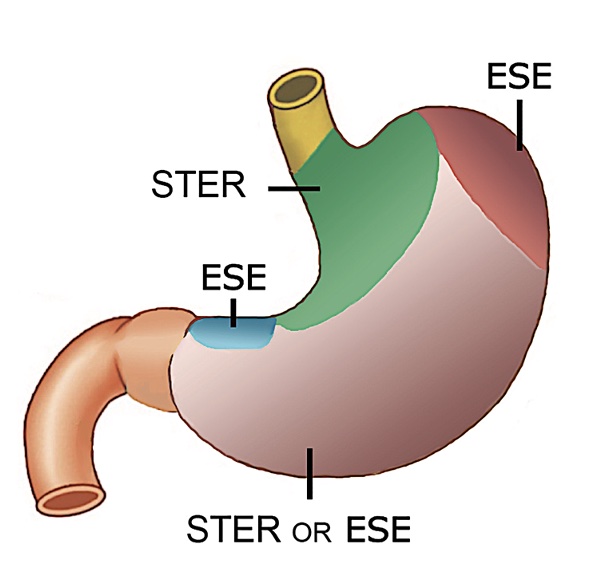
Fig.Ā 7.Per-rectal endoscopic myotomy in a patient with Hirschsprungās disease. (A) Submucosal lifting injection. (B) Transverse mucosal incision 1 to 2 cm above the anal verge. (C) Submucosal tunneling with arrows indicating the muscle layer. (D) Completion of submucosal tunneling. (E) Full-thickness myotomy. (F) Closure of the mucosal incision using endoclips. Courtesy by Mohan Ramchandani (Consultant Gastroenterologist, Asian Institute of Gastroenterology, Hyderabad, India). 
TableĀ 1.Long-term outcomes of per-oral endoscopic myotomy in esophageal motility disorder
TableĀ 2.Outcomes of submucosal tunneling and division of the septum in cases of Zenkerās diverticulum
TableĀ 3.Outcomes of gastric per-oral endoscopic myotomy in refractory gastroparesis
TableĀ 4.Recent updates and future directions on submucosal endoscopy in the gastrointestinal tract POEM, per-oral endoscopic myotomy; PD, pneumatic dilatation; HM, Hellerās myotomy; GERD, gastroesophageal reflux disease; STER, submucosal tunneling endoscopic resection; POET, per-oral endoscopic tumor resection; G-POEM, gastric POEM; EndoFLIP, endoluminal functional lumen imaging probe; PREM, per-rectal endoscopic myotomy; POETRE, per-oral endoscopic tunneling for restoration of the esophagus. REFERENCES1. Sumiyama K, Gostout CJ, Rajan E, et al. Transesophageal mediastinoscopy by submucosal endoscopy with mucosal flap safety valve technique. Gastrointest Endosc 2007;65:679ā683.
2. Sumiyama K, Gostout CJ, Rajan E, et al. Submucosal endoscopy with mucosal flap safety valve. Gastrointest Endosc 2007;65:688ā694.
3. Pasricha PJ, Hawari R, Ahmed I, et al. Submucosal endoscopic esophageal myotomy: a novel experimental approach for the treatment of achalasia. Endoscopy 2007;39:761ā764.
4. Inoue H, Minami H, Kobayashi Y, et al. Peroral endoscopic myotomy (POEM) for esophageal achalasia. Endoscopy 2010;42:265ā271.
5. Nabi Z, Reddy DN. Third space endoscopy: the future of treating gastrointestinal dysmotility. Curr Opin Gastroenterol 2021;37:462ā469.
6. Werner YB, von Renteln D, Noder T, et al. Early adverse events of per-oral endoscopic myotomy. Gastrointest Endosc 2017;85:708ā718.
7. Nabi Z, Reddy DN, Ramchandani M. Adverse events during and after per-oral endoscopic myotomy: prevention, diagnosis, and management. Gastrointest Endosc 2018;87:4ā17.
8. Haito-Chavez Y, Inoue H, Beard KW, et al. Comprehensive analysis of adverse events associated with per oral endoscopic myotomy in 1826 patients: an international multicenter study. Am J Gastroenterol 2017;112:1267ā1276.
9. Nabi Nabi, Talukdar R, Beard R, et al. Outcomes of per-oral endoscopic myotomy in children: a systematic review and meta-analysis. Dysphagia 2022;37:1468ā1481.
10. Zhong C, Huang S, Xia H, et al. Role of peroral endoscopic myotomy in geriatric patients with achalasia: a systematic review and meta-analysis. Dig Dis 2022;40:106ā114.
11. Khan MA, Kumbhari V, Ngamruengphong S, et al. Is POEM the answer for management of spastic esophageal disorders? A systematic review and meta-analysis. Dig Dis Sci 2017;62:35ā44.
12. Nabi Z, Ramchandani M, Chavan R, et al. Peroral endoscopic myotomy in treatment-naĆÆve achalasia patients versus prior treatment failure cases. Endoscopy 2018;50:358ā370.
13. Nabi Z, Ramchandani M, Basha J, et al. Outcomes of per-oral endoscopic myotomy in sigmoid and advanced sigmoid achalasia. J Gastrointest Surg 2021;25:530ā532.
14. Guo H, Yang H, Zhang X, et al. Long-term outcomes of peroral endoscopic myotomy for patients with achalasia: a retrospective single-center study. Dis Esophagus 2017;30:1ā6.
15. HernĆ”ndez MondragĆ³n OV, GonzĆ”lez Martinez MA, Blancas Valencia JM, et al. Long-term quality of life after peroral endoscopic myotomy remains compromised in patients with achalasia type III. Endoscopy 2017;49:1209ā1218.
16. Teitelbaum EN, Dunst CM, Reavis KM, et al. Clinical outcomes five years after POEM for treatment of primary esophageal motility disorders. Surg Endosc 2018;32:421ā427.
17. Liu XY, Cheng J, Chen WF, et al. A risk-scoring system to predict clinical failure for patients with achalasia after peroral endoscopic myotomy. Gastrointest Endosc 2020;91:33ā40.
18. Brewer Gutierrez OI, Moran RA, Familiari P, et al. Long-term outcomes of per-oral endoscopic myotomy in achalasia patients with a minimum follow-up of 4 years: a multicenter study. Endosc Int Open 2020;8:E650āE655.
19. Filicori F, Dunst CM, Sharata A, et al. Long-term outcomes following POEM for non-achalasia motility disorders of the esophagus. Surg Endosc 2019;33:1632ā1639.
20. He C, Li M, Lu B, et al. Long-term efficacy of peroral endoscopic myotomy for patients with achalasia: outcomes with a median follow-up of 36 months. Dig Dis Sci 2019;64:803ā810.
21. Podboy AJ, Hwang JH, Rivas H, et al. Long-term outcomes of per-oral endoscopic myotomy compared to laparoscopic Heller myotomy for achalasia: a single-center experience. Surg Endosc 2021;35:792ā801.
22. Tefas C, BoroČ C, Ciobanu L, et al. POEM: five years of experience in a single east European center. J Gastrointestin Liver Dis 2020;29:323ā328.
23. Nabi Z, Chavan R, Ramchandani M, et al. Long-term outcomes of per-oral endoscopic myotomy in spastic esophageal motility disorders: a large, single-center study. J Clin Gastroenterol 2021;55:594ā601.
24. Onimaru M, Inoue H, Fujiyoshi Y, et al. Long-term clinical results of per-oral endoscopic myotomy (POEM) for achalasia: first report of more than 10-year patient experience as assessed with a questionnaire-based survey. Endosc Int Open 2021;9:E409āE416.
25. McKay SC, Dunst CM, Sharata AM, et al. POEM: clinical outcomes beyond 5 years. Surg Endosc 2021;35:5709ā5716.
26. Modayil RJ, Zhang X, Rothberg B, et al. Peroral endoscopic myotomy: 10-year outcomes from a large, single-center U.S. series with high follow-up completion and comprehensive analysis of long-term efficacy, safety, objective GERD, and endoscopic functional luminal assessment. Gastrointest Endosc 2021;94:930ā942.
27. Campagna RA, Cirera A, Holmstrom AL, et al. Outcomes of 100 patients more than 4 years after POEM for achalasia. Ann Surg 2021;273:1135ā1140.
28. Sanaka MR, Chadalavada P, Covut F, et al. Clinical success and correlation of Eckardt scores with barium esophagram after peroral endoscopic myotomy in achalasia. J Gastrointest Surg 2021;25:278ā281.
29. Xu S, Chai N, Tang X, et al. Outcomes of peroral endoscopic myotomy in challenging achalasia patients: a long-term follow-up study. Surg Endosc 2021;35:3732ā3743.
30. Andolfi C, Fisichella PM. Meta-analysis of clinical outcome after treatment for achalasia based on manometric subtypes. Br J Surg 2019;106:332ā341.
31. Ponds FA, Fockens P, Lei A, et al. Effect of peroral endoscopic myotomy vs pneumatic dilation on symptom severity and treatment outcomes among treatment-naĆÆve patients with achalasia: a randomized clinical trial. JAMA 2019;322:134ā144.
32. Werner YB, Hakanson B, Martinek J, et al. Endoscopic or surgical myotomy in patients with idiopathic achalasia. N Engl J Med 2019;381:2219ā2229.
33. Jung HK, Hong SJ, Lee OY, et al. 2019 Seoul consensus on esophageal achalasia guidelines. J Neurogastroenterol Motil 2020;26:180ā203.
34. Khashab MA, Vela MF, Thosani N, et al. ASGE guideline on the management of achalasia. Gastrointest Endosc 2020;91:213ā227.
35. Oude Nijhuis RA, Zaninotto G, Roman S, et al. European guidelines on achalasia: United European Gastroenterology and European Society of Neurogastroenterology and Motility recommendations. United European Gastroenterol J 2020;8:13ā33.
36. Vaezi MF, Pandolfino JE, Yadlapati RH, et al. ACG clinical guidelines: diagnosis and management of achalasia. Am J Gastroenterol 2020;115:1393ā1411.
37. Weusten BL, Barret M, Bredenoord AJ, et al. Endoscopic management of gastrointestinal motility disorders - part 1: European Society of Gastrointestinal Endoscopy (ESGE) guideline. Endoscopy 2020;52:498ā515.
38. Repici A, Cappello A, Spadaccini M, et al. Cap-assisted endoscopic septotomy of Zenker's diverticulum: early and long-term outcomes. Am J Gastroenterol 2021;116:1853ā1858.
39. Costamagna G, Iacopini F, Bizzotto A, et al. Prognostic variables for the clinical success of flexible endoscopic septotomy of Zenker's diverticulum. Gastrointest Endosc 2016;83:765ā773.
40. Huberty V, El Bacha S, Blero D, et al. Endoscopic treatment for Zenker's diverticulum: long-term results (with video). Gastrointest Endosc 2013;77:701ā707.
41. Zhang LY, Nieto J, Ngamruengphong S, et al. Zenker's diverticulum: advancing beyond the tunnel. VideoGIE 2021;6:562ā567.
42. Repici A, Spadaccini M, Belletrutti PJ, et al. Peroral endoscopic septotomy for short-septum Zenker's diverticulum. Endoscopy 2020;52:563ā568.
43. Yang J, Novak S, Ujiki M, et al. An international study on the use of peroral endoscopic myotomy in the management of Zenker's diverticulum. Gastrointest Endosc 2020;91:163ā168.
44. Budnicka A, Januszewicz W, BiaÅek AB, et al. Peroral endoscopic myotomy in the management of Zenker's diverticulum: a retrospective multicenter study. J Clin Med 2021;10:187.
45. Elkholy S, El-Sherbiny M, Delano-Alonso R, et al. Peroral endoscopic myotomy as treatment for Zenker's diverticulum (Z-POEM): a multi-center international study. Esophagus 2021;18:693ā699.
46. Sanaei O, Ichkhanian Y, MondragĆ³n OV, et al. Impact of prior treatment on feasibility and outcomes of Zenker's peroral endoscopic myotomy (Z-POEM). Endoscopy 2021;53:722ā726.
47. Mittal C, Diehl DL, Draganov PV, et al. Practice patterns, techniques, and outcomes of flexible endoscopic myotomy for Zenker's diverticulum: a retrospective multicenter study. Endoscopy 2021;53:346ā353.
48. Al Ghamdi SS, Farha J, Moran RA, et al. Zenker's peroral endoscopic myotomy, or flexible or rigid septotomy for Zenker's diverticulum: a multicenter retrospective comparison. Endoscopy 2022;54:345ā351.
49. Kahaleh M, Mahpour NY, Tyberg A, et al. Per oral endoscopic myotomy for Zenker's diverticulum: a novel and superior technique compared with septotomy? J Clin Gastroenterol 2022;56:224ā227.
50. Yang J, Zeng X, Yuan X, et al. An international study on the use of peroral endoscopic myotomy (POEM) in the management of esophageal diverticula: the first multicenter D-POEM experience. Endoscopy 2019;51:346ā349.
51. Basile P, Gonzalez JM, Le Mouel JP, et al. Per-oral endoscopic myotomy with septotomy for the treatment of distal esophageal diverticula (D-POEM). Surg Endosc 2020;34:2321ā2325.
52. Nabi Z, Chavan R, Asif S, et al. Per-oral endoscopic myotomy with division of septum (D-POEM) in epiphrenic esophageal diverticula: outcomes at a median follow-up of two years. Dysphagia 2022;37:839ā847.
53. Demeter M, ÄuriÄek M, VorÄĆ”k M, et al. S-POEM in treatment of achalasia and esophageal epiphrenic diverticula - single center experience. Scand J Gastroenterol 2020;55:509ā514.
54. Kinoshita M, Tanaka S, Kawara F, et al. Peroral endoscopic myotomy alone is effective for esophageal motility disorders and esophageal epiphrenic diverticulum: a retrospective single-center study. Surg Endosc 2020;34:5447ā5454.
55. Samanta J, Nabi Z, Dhar J, et al. Peroral endoscopic myotomy (POEM) for esophageal diverticula. Minerva Gastroenterol (Torino) 2021 Sep 13 [Epub]. https://doi.org/10.23736/S2724-5985.21.02984-3.
56. Wagh MS, Yang D, Chavalitdhamrong D, et al. Per-oral endoscopic tunneling for restoration of the esophagus (POETRE). Gastrointest Endosc 2014;80:330.
57. Wagh MS, Draganov PV. Per-oral endoscopic tunneling for restoration of the esophagus: a novel endoscopic submucosal dissection technique for therapy of complete esophageal obstruction. Gastrointest Endosc 2017;85:722ā727.
58. FĆ©lix C, Barreiro P, Rodrigues Azevedo J, et al. Per-oral endoscopic tunneling for restoration of the esophagus (POETRE) in the management of a complete esophageal obstruction. Endosc Int Open 2021;9:E1084āE1085.
59. Toro JP, Lytle NW, Patel AD, et al. Efficacy of laparoscopic pyloroplasty for the treatment of gastroparesis. J Am Coll Surg 2014;218:652ā660.
60. Shada AL, Dunst CM, Pescarus R, et al. Laparoscopic pyloroplasty is a safe and effective first-line surgical therapy for refractory gastroparesis. Surg Endosc 2016;30:1326ā1332.
61. Brown AM, Pryor AD, Docimo S Jr. Per oral pyloromyotomy utilizing a lesser curvature approach: how we do it. Surg Endosc 2020;34:5168ā5171.
62. Abdelfatah MM, Li B, Kapil N, et al. Short-term outcomes of double versus single pyloromyotomy at peroral endoscopic pyloromyotomy in the treatment of gastroparesis (with video). Gastrointest Endosc 2020;92:603ā609.
63. Ichkhanian Y, Vosoughi K, Aghaie Meybodi M, et al. Comprehensive analysis of adverse events associated with gastric peroral endoscopic myotomy: an international multicenter study. Surg Endosc 2021;35:1755ā1764.
64. Mekaroonkamol P, Patel V, Shah R, et al. Association between duration or etiology of gastroparesis and clinical response after gastric per-oral endoscopic pyloromyotomy. Gastrointest Endosc 2019;89:969ā976.
65. Gregor L, Wo J, DeWitt J, et al. Gastric peroral endoscopic myotomy for the treatment of refractory gastroparesis: a prospective single-center experience with mid-term follow-up (with video). Gastrointest Endosc 2021;94:35ā44.
66. Abdelfatah MM, Noll A, Kapil N, et al. Long-term outcome of gastric per-oral endoscopic pyloromyotomy in treatment of gastroparesis. Clin Gastroenterol Hepatol 2021;19:816ā824.
67. Ragi O, Jacques J, Branche J, et al. One-year results of gastric peroral endoscopic myotomy for refractory gastroparesis: a French multicenter study. Endoscopy 2021;53:480ā490.
68. Vosoughi K, Ichkhanian Y, Benias P, et al. Gastric per-oral endoscopic myotomy (G-POEM) for refractory gastroparesis: results from an international prospective trial. Gut 2022;71:25ā33.
69. Labonde A, Lades G, Debourdeau A, et al. Gastric peroral endoscopic myotomy in refractory gastroparesis: long-term outcomes and predictive score to improve patient selection. Gastrointest Endosc 2022;96:500ā508.
70. Spadaccini M, Maselli R, Chandrasekar VT, et al. Gastric peroral endoscopic pyloromyotomy for refractory gastroparesis: a systematic review of early outcomes with pooled analysis. Gastrointest Endosc 2020;91:746ā752.
71. Pioppo L, Reja D, Gaidhane M, et al. Gastric per-oral endoscopic myotomy versus pyloromyotomy for gastroparesis: an international comparative study. J Gastroenterol Hepatol 2021;36:3177ā3182.
72. Mohan BP, Chandan S, Jha LK, et al. Clinical efficacy of gastric per-oral endoscopic myotomy (G-POEM) in the treatment of refractory gastroparesis and predictors of outcomes: a systematic review and meta-analysis using surgical pyloroplasty as a comparator group. Surg Endosc 2020;34:3352ā3367.
73. Shen S, Luo H, Vachaparambil C, et al. Gastric peroral endoscopic pyloromyotomy versus gastric electrical stimulation in the treatment of refractory gastroparesis: a propensity score-matched analysis of long term outcomes. Endoscopy 2020;52:349ā358.
74. Lv XH, Wang CH, Xie Y. Efficacy and safety of submucosal tunneling endoscopic resection for upper gastrointestinal submucosal tumors: a systematic review and meta-analysis. Surg Endosc 2017;31:49ā63.
75. Chen Y, Wang M, Zhao L, et al. The retrospective comparison between submucosal tunneling endoscopic resection and endoscopic submucosal excavation for managing esophageal submucosal tumors originating from the muscularis propria layer. Surg Endosc 2020;34:417ā428.
76. Ponte Neto FL, de Moura DT, Sagae VM, et al. Endoscopic resection of esophageal and gastric submucosal tumors from the muscularis propria layer: submucosal tunneling endoscopic resection versus endoscopic submucosal excavation: a systematic review and meta-analysis. Surg Endosc 2021;35:6413ā6426.
77. Lu J, Jiao T, Li Y, et al. Heading toward the right direction: solution package for endoscopic submucosal tunneling resection in the stomach. PLoS One 2015;10:e0119870.
78. Tan Y, Lv L, Duan T, et al. Comparison between submucosal tunneling endoscopic resection and video-assisted thoracoscopic surgery for large esophageal leiomyoma originating from the muscularis propria layer. Surg Endosc 2016;30:3121ā3127.
79. Chai N, Du C, Gao Y, et al. Comparison between submucosal tunneling endoscopic resection and video-assisted thoracoscopic enucleation for esophageal submucosal tumors originating from the muscularis propria layer: a randomized controlled trial. Surg Endosc 2018;32:3364ā3372.
80. Onimaru M, Inoue H, Bechara R, et al. Clinical outcomes of per-oral endoscopic tumor resection for submucosal tumors in the esophagus and gastric cardia. Dig Endosc 2020;32:328ā336.
81. Bapaye A, Dashatwar P, Biradar V, et al. Initial experience with per-rectal endoscopic myotomy for Hirschsprung's disease: medium and long term outcomes of the first case series of a novel third-space endoscopy procedure. Endoscopy 2021;53:1256ā1260.
82. Nabi Z, Shava U, Sekharan A, et al. Diagnosis of Hirschsprung's disease in children: preliminary evaluation of a novel endoscopic technique for rectal biopsy. JGH Open 2018;2:322ā326.
83. Nabi Z, Chavan R, Shava U, et al. A novel endoscopic technique to obtain rectal biopsy specimens in children with suspected Hirschsprung's disease. VideoGIE 2018;3:157ā158.
84. Li L, Chai N, Linghu E, et al. Safety and efficacy of using a short tunnel versus a standard tunnel for peroral endoscopic myotomy for ling type IIc and III achalasia: a retrospective study. Surg Endosc 2019;33:1394ā1402.
85. Huang S, Ren Y, Peng W, et al. Peroral endoscopic shorter versus longer myotomy for the treatment of achalasia: a comparative retrospective study. Esophagus 2020;17:477ā483.
86. Gu L, Ouyang Z, Lv L, et al. Safety and efficacy of peroral endoscopic myotomy with standard myotomy versus short myotomy for treatment-naĆÆve patients with type II achalasia: a prospective randomized trial. Gastrointest Endosc 2021;93:1304ā1312.
87. Nabi Z, Ramchandani M, Sayyed M, et al. Comparison of short versus long esophageal myotomy in cases with idiopathic achalasia: a randomized controlled trial. J Neurogastroenterol Motil 2021;27:63ā70.
88. Repici A, Fuccio L, Maselli R, et al. GERD after per-oral endoscopic myotomy as compared with Heller's myotomy with fundoplication: a systematic review with meta-analysis. Gastrointest Endosc 2018;87:934ā943.
89. Sanaka MR, Thota PN, Parikh MP, et al. Peroral endoscopic myotomy leads to higher rates of abnormal esophageal acid exposure than laparoscopic Heller myotomy in achalasia. Surg Endosc 2019;33:2284ā2292.
90. Karyampudi A, Nabi Z, Ramchandani M, et al. Gastroesophageal reflux after per-oral endoscopic myotomy is frequently asymptomatic, but leads to more severe esophagitis: a case-control study. United European Gastroenterol J 2021;9:63ā71.
91. Nabi Z, Ramchandani M, Kotla R, et al. Gastroesophageal reflux disease after peroral endoscopic myotomy is unpredictable, but responsive to proton pump inhibitor therapy: a large, single-center study. Endoscopy 2020;52:643ā651.
92. Nabi Z, Talukdar R, Mandavdhare H, et al. Short versus long esophageal myotomy during peroral endoscopic myotomy: a systematic review and meta-analysis of comparative trials. Saudi J Gastroenterol 2022;28:261ā267.
93. Knowles TB, Jackson AS, Chang SC, et al. Changes in distensibility index during an incremental POEM myotomy. J Gastrointest Surg 2022;26:1140ā1146.
94. Inoue H, Ueno A, Shimamura Y, et al. Peroral endoscopic myotomy and fundoplication: a novel NOTES procedure. Endoscopy 2019;51:161ā164.
95. Nabi Z, Ramchandani M, Reddy DN. Per-oral endoscopic myotomy and gastroesophageal reflux: where do we stand after a decade of "POETRY"? Indian J Gastroenterol 2019;38:287ā294.
96. Tanaka S, Toyonaga T, Kawara F, et al. Novel per-oral endoscopic myotomy method preserving oblique muscle using two penetrating vessels as anatomic landmarks reduces postoperative gastroesophageal reflux. J Gastroenterol Hepatol 2019;34:2158ā2163.
97. Nabi Z, Ramchandani M, Darisetty S, et al. Peroral endoscopic myotomy with endoscopic fundoplication in a patient with idiopathic achalasia. Endoscopy 2020;52:74ā75.
98. Tyberg A, Choi A, Gaidhane M, et al. Transoral incisional fundoplication for reflux after peroral endoscopic myotomy: a crucial addition to our arsenal. Endosc Int Open 2018;6:E549āE552.
99. Su B, Callahan ZM, Novak S, et al. Using impedance planimetry (EndoFLIP) to evaluate myotomy and predict outcomes after surgery for achalasia. J Gastrointest Surg 2020;24:964ā971.
100. Vosoughi K, Ichkhanian Y, Jacques J, et al. Role of endoscopic functional luminal imaging probe in predicting the outcome of gastric peroral endoscopic pyloromyotomy (with video). Gastrointest Endosc 2020;91:1289ā1299.
101. Chen T, Xu A, Lian J, et al. Transcolonic endoscopic appendectomy: a novel natural orifice transluminal endoscopic surgery (NOTES) technique for the sessile serrated lesions involving the appendiceal orifice. Gut 2021;70:1812ā1814.
102. Liu XY, Li QL, Xu XY, et al. Endoscopic transgastric cholecystectomy: a novel approach for minimally invasive cholecystectomy. Endoscopy 2021;53:E50āE51.
103. Ikebuchi Y, Kanda T, Ikeda H, et al. Identification of human herpes virus 1 encoded microRNAs in biopsy samples of lower esophageal sphincter muscle during peroral endoscopic myotomy for esophageal achalasia. Dig Endosc 2020;32:136ā142.
104. Chen S, Zhang M, Liang M, et al. The number of interstitial cells of cajal differs among different subtypes of achalasia and is related to patients' prognosis. Clin Transl Gastroenterol 2021;12:e00388.
105. Reddy CA, Law R, Appelman HD, et al. Per-oral endoscopic myotomy biopsies of achalasia patients reveal schwann cell depletion in the muscularis propria. Clin Gastroenterol Hepatol 2021;19:1294ā1295.
106. Chen H, Calderon LF, Shah R, et al. Simultaneous examination of eosinophil infiltration in esophageal mucosa and muscle in patients with achalasia: direct biopsy of the esophageal muscle at per-oral endoscopic myotomy. Dig Dis Sci 2022;67:170ā176.
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
