Effective endoscopic band ligation for diverticular perforation with a refractory pelvic abscess
Article information
Diverticulitis is the most common diverticular disease. Its mainstream treatment is a nonsurgical approach involving bowel rest and broad-spectrum antibiotic administration. However, severe acute diverticulitis can result in perforation, abscess, and fistula formation, which may require surgery.1 Pelvic abscess (PA), a frequent complication of abdominal bacterial infections, is defined as a mass filled with necrotic and purulent material demarcated by a thick fibrous wall, which is formed by an exudative response of the surrounding tissues.2 Considering that PA is associated with long-term sequelae and significant morbidity and mortality,1 appropriate treatment, not only for PA, but also for its underlying condition is crucial.
The authors received approval from the Institutional Review Board from Omihachiman Community Medical Center (ERB-C-875), and informed consent was obtained from the individual involved in this report.
Here we report the case of a 49-year-old Japanese woman who was admitted to our hospital for hypogastric pain due to refractory and uncontrollable PA. On the day of admission, abdominal computed tomography revealed a PA (112.4×110 mm in size) with a large amount of free air (Fig. 1A). Initially, we considered gastrointestinal perforation; however, no obvious perforation site could be detected. As the abscess cavity was located in the extra-abdominal cavity anterior to the uterus, we considered PA of gynecological origin as a differential diagnosis. Hence, after discussions among the gastroenterology, surgery, and gynecology services, we performed transvaginal drainage on hospitalization day 1; however, the clinical course did not improve. On day 5, amidotrizoic acid enema revealed that the PA was connected to a diverticular perforation (Fig. 1B) of the sigmoid colon. Computed tomography colonography comprising images after amidotrizoic acid enema showed a PA extending from the perforated diverticulum (Fig. 1C). Despite transvaginal drainage and two sessions of endoscopic ultrasound-guided pelvic abscess drainage (EUS-PAD) and double-barrel ileostomy, the PA did not show any improvement. Hence, colonoscopy was performed on day 28, revealing adherent pus (Fig. 2A) at the diverticular perforation site (Fig. 2B), which was identified and marked with endoclips before the colonoscope was removed. Placement of an endoscopic retrograde cholangiopancreatography cannula close to the perforation concurrent with injection of contrast agent confirmed a continuous channel from the perforation to the abscess (Fig. 2C). A colonoscope with an endoscopic band ligation (EBL) device was then reinserted into the target diverticulum. The target diverticulum was suctioned into the EBL cap and the elastic band was released to close the perforation (Fig. 2D). Although an additional EUS-PAD was required for residual PA after EBL, the patient’s condition subsequently improved, and she was followed up on an outpatient basis. Four double-pigtail plastic stents inserted for EUS-PAD (Fig. 3A) were endoscopically removed using grasping forceps (Fig. 3B, C).
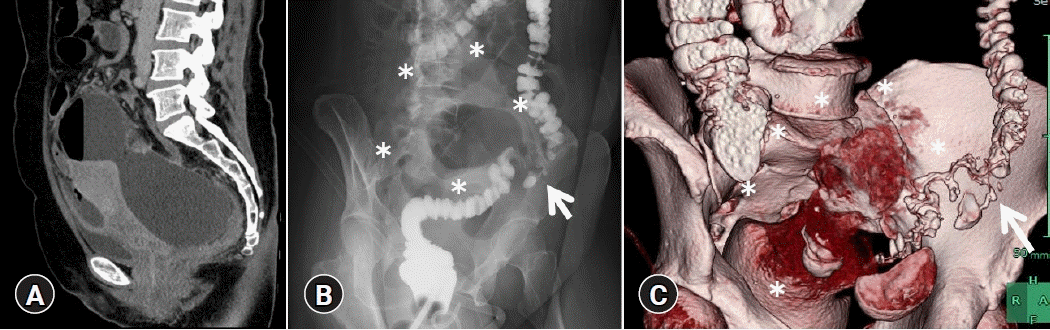
(A) Abdominal computed tomography showing a 112.4×110 mm pelvic abscess caused by a diverticular perforation in the sigmoid colon. (B) On day 5, amidotrizoic acid enema revealed a pelvic abscess (asterisks) connected to a diverticular perforation (arrow) in the sigmoid colon. (C) Computed tomography colonography comprising images after amidotrizoic acid enema showing a pelvic abscess (asterisks) extending from the perforated diverticulum (arrow).

(A) Endoscopy showing adherent pus at the diverticular perforation site. (B) A perforated diverticulum (arrow) was identified and marked with endoclips prior to colonoscope removal. (C) When an endoscopic retrograde cholangiopancreatography cannula was placed close to the perforation and contrast medium was injected, a continuous channel (arrow) from the perforation to the abscess was confirmed. (D) The target diverticulum was suctioned into the band ligator cap, and the elastic band was released to close the perforation site.
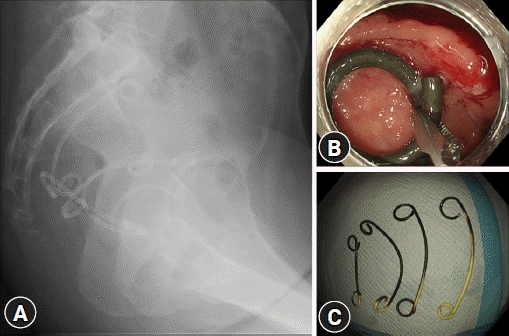
Four double-pigtail plastic stents, inserted for endoscopic ultrasound-guided pelvic abscess drainage (A), were endoscopically removed with grasping forceps (B, C).
Manabe et al.3 have reported that the 16% of patients with colonic diverticulitis with complications such as abscesses had a mortality rate of 2.8%. In contrast, the mortality rate in the absence of complications was 0.2%. This suggests that aggressive intervention is crucial for patients with diverticular perforation complicated by abscesses or other conditions. Regarding PA drainage, Ballard et al.4 reported a 94% success rate with both transvaginal and transrectal drainage. Additionally, these approaches have been strongly favored over the more painful transgluteal approaches in clinical experience. Regarding endoscopic interventions, in 2003, Giovannini et al.5 presented a promising novel treatment modality using EUS-PAD; this showed several advantages over conventional surgery, and the percutaneous interventional procedure was thus developed. Endoscopic ultrasound provides the shortest route to the puncture site, minimizing the risk of puncturing other organs or vessels. Moreover, Dhindsa et al.6 found in a meta-analysis that EUS-PAD has high technical (100%) and clinical success (92%) rates with low occurrence of adverse events.
In our patient, we identified the site of the diverticular perforation and subsequently performed EBL at the same site, with the patient showing progressive improvement. Management of PA with diverticular perforation was critical. Colonic fistula is a common complication of acute diverticulitis. We considered that the fistula was caused by the formation of an abscess with repeated colonic diverticulitis, which resulted in inflammatory adhesions. Ramesh et al.7 reported that diverticular abscesses managed using EUS-PAD had significantly lower treatment success rates than abscesses of other etiologies. They speculated that the frequently multiloculated and highly viscous nature of diverticular abscesses results in increased treatment failure rates. Although technical success with EUS-PAD can be expected, if the causative disease has not been cured, the diverticular perforation will not close.
Careful consideration should be given to the size, etiology, and maturity of the PA when applying EUS-PAD. A smaller PA (<4 cm) may respond to simple aspiration, and can be usually treated without endoscopic intervention, whereas a larger PA would benefit from drainage device placement.8 Considerations should also be taken for PA of diverticular etiology as higher rates of complications are seen in such cases.7 EUS-PAD should be strictly avoided in patients with ascites or if the PA is >20 mm from the bowel lumen.8
EBL has recently been reported as a useful hemostatic therapy for colonic diverticular and hemorrhoidal bleeding because of its safety and low rate of rebleeding.9 In our case, we successfully used the EBL technique, which is commonly used in continuous diverticular bleeding, for diverticular perforation. The EBL technique is useful if the perforation site can be identified in difficult-to-treat patients with diverticular perforation. In our case, although the EBL was able to seal the entrance to the PA, the residual contents of the abscess could have caused recurrence; therefore, another EUS-PAD was performed. Thereafter, the patient’s clinical course improved.
Treatment of diverticular hemorrhage with EBL is widely recognized; however, this carries a risk of diverticular perforation, albeit infrequently. Kobayashi et al.10 reviewed cases of colonic diverticular hemorrhage that were treated with EBL, and reported that perforation occurred in two cases (0.31%).
In conclusion, EBL is beneficial in patients with uncontrollable diverticular perforation if the perforation site can be identified. Although EBL cannot be applied to all patients with colonic diverticular perforation, it is expected that the use of this method, which has several advantages over surgery, will gradually increase in the future. Furthermore, regarding PA treatment, it is necessary to not only consider how to drain the PA, but also to discuss with experts a treatment for the causative disease. We suggest a multidisciplinary discussion of PA management, as all experts are stakeholders in decision-making and treatment.
Notes
Conflicts of Interest
The authors have no potential conflicts of interest.
Funding
None.
Author Contributions
Conceptualization: KS; Data curation: KS; Formal analysis: KS; Investigation: KS; Methodology: KS; Project administration: KS; Resources: KS; Supervision: KS; Validation: KS; Visualization: KS; Writing–original draft: KS; Writing–review & editing: KS, AM.
