Gastric cancer presenting with ramucirumab-related gastrocolic fistula successfully managed by colonic stenting: a case report
Article information
Abstract
We report a rare case of gastric cancer presenting with a gastrocolic fistula during ramucirumab and paclitaxel combination therapy that was successfully managed with colonic stenting. A 75-year-old man was admitted to our hospital with the chief complaint of melena. Esophagogastroduodenoscopy revealed a large ulcerated tumor in the lower stomach, judged by laparoscopy as unresectable (sT4bN1M0). After four cycles of first-line chemotherapy with S-1 plus oxaliplatin, the patient showed disease progression, and second-line therapy with ramucirumab and paclitaxel was started. At the end of the third cycle, the patient had gastric antral stenosis, which necessitated the placement of a gastroduodenal stent. When the patient complained of diarrhea 10 days later, esophagogastroduodenoscopy revealed a fistula between the greater curvature of the stomach and the transverse colon. The fistula was covered by double colonic stenting, with a covered metal stent placed within an uncovered metal stent, after which leakage from the stomach to the colon stopped.
INTRODUCTION
Ramucirumab (RAM) is a human IgG1 monoclonal antibody against vascular endothelial growth factor receptor 2.1,2 Although improvements in the overall survival and progression-free survival in patients with gastric cancer have been achieved by RAM alone or in combination with paclitaxel (PTX),3,4 RAM also carries the risk of causing adverse events, including gastrointestinal (GI) perforation.5 Here, we report a case of gastric cancer that presented with a gastrocolic fistula during treatment with RAM plus PTX, which was successfully managed with double colonic stenting.
CASE REPORT
A 75-year-old man was admitted to our hospital with a chief complaint of melena. The patient had chronic heart failure, palmoplantar pustulosis, osteoarthritis, and type 2 diabetes mellitus. The patient had been receiving low-dose aspirin (LDA) (100 mg/day), warfarin (4 mg/day), rabeprazole (10 mg/day), furosemide (10 mg/day), carvedilol (5 mg/day), prednisolone (5 mg/day), and teneligliptin (20 mg/day). He was in a state of pre-shock with an elevated heart rate and low blood pressure on admission, accompanied by a low hemoglobin level of 7.5 g/dL. Urgent esophagogastroduodenoscopy (EGD) revealed a hemorrhagic cancer in the gastric antrum (Fig. 1A), which was pathologically confirmed as moderately differentiated adenocarcinoma, positive for human epidermal growth factor receptor 2. Although enhanced computed tomography (CT) showed no evidence of distant metastasis other than the primary lesion, which was 8 cm in size (Fig. 1B), diagnostic laparoscopy revealed direct invasion of the liver, pancreas, and transverse mesocolon, rendering the diagnosis of unresectable advanced gastric cancer (sT4bN1M0 Stage IIIb).

Features of primary gastric cancer before and during chemotherapy. (A) Esophagogastroduodenoscopy before the application of chemotherapy showed advanced gastric cancer at the anterior wall of the gastric antrum. (B) Enhanced computed tomography before the application of chemotherapy showed advanced gastric cancer at the gastric antrum. (C) Esophagogastroduodenoscopy after three cycles of ramucirumab plus paclitaxel. (D) Radiograph after three cycles of ramucirumab plus paclitaxel.
The patient received first-line chemotherapy with SOX (S-1, 80 mg/day, days 1–14 plus oxaliplatin, 120 mg/day, day 1), but not trastuzumab, because of chronic heart failure. He also received concomitant radiotherapy (50 Gy/25 Fr) to control the bleeding from the primary lesion. Although two cycles of SOX yielded a partial response, oxaliplatin was discontinued because of grade 2 peripheral neuropathy. When the disease progressed after four cycles of S-1 monotherapy, RAM (8 mg/kg, days 1 and 15) plus PTX (80 mg/body surface area [m2], days 1, 8, and 15) was selected as second-line chemotherapy.
After three cycles of RAM plus PTX, the patient complained of anorexia and nausea with the progression of anemia. EGD and fluoroscopy revealed an enlarged primary tumor causing antral stenosis (Fig. 1C, D), for which we placed a gastroduodenal metal stent (90×22 mm, Evolution; Cook Medical) (Fig. 2A). Although the anorexia and nausea improved, the patient complained of feculent vomiting and severe diarrhea 10 days after the placement of the gastroduodenal metal stent. The patient’s stool contained undigested enteral nutrition that was administered via a nasogastric feeding tube. Plain CT revealed an air-dense area indicating a fistulous tract between the stomach and transverse colon (Fig. 2B). This was confirmed by EGD as an elliptical fistula in the large well-demarcated ulcer at the greater curvature, through which the mucosa of the transverse colon was observed (Fig. 2C). Colonoscopy (Fig. 2D) and fluoroscopy (Fig. 3A) also revealed a fistula located at a distance from the proximal edge of the gastroduodenal stent. Surgical repair of the gastrocolic fistula was impossible because of the unresectability of the advanced primary tumor and poor performance status of the patient at that time. The patient was informed of the potential risks of colonic stenting, such as migration and GI perforation, and underwent colonic metal stent placement to cover the gastrocolic fistula. To avoid dislodgement of the stent, we first placed an uncovered metal stent (120×22 mm, Niti-S; TaeWoong Medical) (Fig. 3B), followed by a covered metal stent (120×22 mm, Niti-S) inside the uncovered stent to cover the gastrocolic fistula. There were no procedure-related complications, and fluoroscopy showed minimal gastrographin leakage from the stomach to the colon (Fig. 3C). Feculent vomiting and diarrhea dramatically improved, and the patient was able to resume oral intake of a semi‐digestion nutritional agent (approximately 900 kcal/day) five days after the procedure. A follow-up radiographic examination at two months later showed no signs of stent migration. He chose to withdraw from chemotherapy and opted for the best supportive care. Oral intake became impossible three months after stent placement because of disease progression. However, the patient had no recurrence of feculent vomiting, diarrhea, or significant abdominal symptoms until he died approximately five months after the diagnosis of gastrocolic fistula, which was one year and five months after the initial diagnosis.
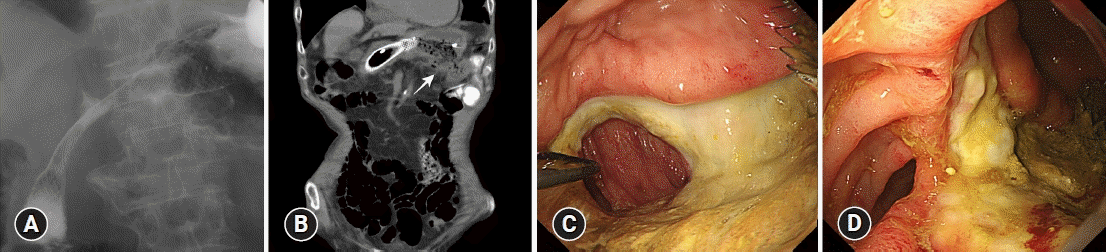
Gastroduodenal stent placement for antral stenosis and appearance of gastrocolic fistula. (A) Radiography showed successful placement of gastric metallic stent for antral stenosis. (B) Computed tomography at 10 days after the placement of gastric metallic stent revealed a fistula (arrow) between the stomach and transverse colon. (C) Esophagogastroduodenoscopy showed gastrocolic fistula. (D) Colonoscopy showed gastrocolic fistula.

Placement of a double colonic metal stent to cover the gastrocolic fistula. (A) Fluoroscopy showed a gastrocolic fistula. (B) Fluoroscopy showed an uncovered colonic metal stent placed in the transverse colon. (C) Fluoroscopy showed little leakage from the stomach to the transverse colon after stent-in-stent placement of a covered stent.
DISCUSSION
We report the first case of gastric cancer presenting with a RAM-associated gastrocolic fistula, which is also the first case of a gastrocolic fistula that was successfully treated by placing a covered colonic metal stent in combination with an uncovered stent that functioned for several months with no procedure-related complications.
Although GI perforation is a rare complication of chemotherapy for gastric cancer, gastrocolic fistulas are even rarer. The REGARD and RAINBOW trials showed no significant increase in the GI perforation in the RAM-treated group, with the highest incidence of 1.2% in the RAINBOW trial3,4; however, the incidence of GI perforation is higher in real-world settings. For instance, in a Korean cohort study, seven of 228 patients (3.1%) who received RAM plus PTX for gastric cancer developed GI perforation.6 In a meta-analysis including 4,579 patients with solid organ malignancies, RAM was associated with a 1.5% incidence of GI perforation and calculated relative risk of 2.56 (95% confidence interval, 1.29–5.09).5
There are two theories associated with the formation of gastrocolic fistulas.7,8 One theory is that the fistula is caused by the collapse of tumor cells that directly invade the gastrocolic omentum by necrosis or mechanical pressure.6 The other is that the fistula is formed by inflammatory peritoneal reactions possibly associated with delayed wound healing. RAM, possibly in a similar way as bevacizumab,9 could be one of the causative factors of these processes leading to fistula formation, as well as other factors, such as radiation,10,11 steroids,12 and LDA.13 In the present case, with all these potential factors involved, it seems that both theories were involved in the formation of the gastrocolic fistula. The tumor tissue invading the colon might have become necrotic due to chemotherapy; additionally, tissue friability and delayed wound healing may have been caused by the effects of radiotherapy and long-term use of steroids and LDA. Although there might have been increased gastric pressure even after gastroduodenal stent placement for antral stenosis, direct mechanical damage caused by the metallic stent itself was unlikely, because the fistula was distant from the edge of the stent. Therefore, we considered RAM, in conjunction with other factors, such as radiotherapy and steroids, as the cause of gastrocolic fistula.
As for the treatment options to manage gastrocolic fistula other than surgery,14 we chose stent placement over other endoscopic procedures, such as over-the-scope clips, clipping with thread, or fibrin glue filling. This was because the fistula measured 3 to 4 centimeters in width and formed in a large ulcer bed. A rare case of successful treatment with colonic covered stent placement for a gastrocolic fistula secondary to gastric cancer has been reported.15 In this report, only a covered stent was placed because colonic stenosis at the transverse colon was severe. However, our case differed in that the severity of colonic stenosis was moderate. Therefore, we first performed colonic stenting to cover the fistula using an uncovered metal stent to prevent migration, followed by a stent-in-stent placement of a covered stent to reduce fistula leakage. As a result, the double stents remained in the correct place, with no recurrence of feculent vomiting or severe diarrhea.
In conclusion, this is the first case of gastric cancer with RAM-related gastrocolic fistula. We should keep in mind that RAM has a risk of causing gastrocolic fistula. Colonic stenting can be an option for managing such fistulas.
Notes
Conflicts of Interest
Eikichi Ihara participated in the funded research of Takeda Pharmaceutical Co., Ltd. and belonged to an endowed course supported by companies, including Ono Pharmaceutical Co, Ltd, Miyarisan Pharmaceutical Co., Ltd., Sanwa Kagaku Kenkyusho Co., Ltd., Otsuka Pharmaceutical Factory Inc., Fujifilm Medical Co., Ltd., Terumo Corporation, Fancl Corporation, and Ohga Pharmacy. The authors have no potential conflicts of interest.
Funding
None.
Acknowledgments
We thank Cathel Kerr, BSc, PhD, and Edanz Group (https://en-author-services.edanz.com/ac) for editing the draft of this manuscript.
Author Contributions
Conceptualization: HF; Investigation: MW, HF, YI, YS; Supervision: NH, MN, EI; Writing–original draft: HF; Writing–review & editing: all authors.
